| | |
| 5 | | Executive Compensation Matters (continued) |
In May 2017 FAMPYRA was approved for walking improvement in people with MS by the EC.
In August 2017 IMRALDI, an adalimumab biosimilar referencing HUMIRA developed through our joint venture, Samsung Bioepis, was approved by the EC.
Clinical Trials
MS and Neuroimmunology
In January 2017September 2018 we completed enrollment of the Phase 2b AFFINITY study evaluating opicinumab, anti-LINGO, as anadd-on therapy in MS patients who are adequately controlled on their anti-inflammatory disease-modifying therapy (DMT), versus the DMT alone.
In November 2018 we initiated athe Phase 1 trial3b NOVA study evaluating the efficacy and safety of BIIB076, ananti-tau monoclonal antibody,extended interval dosing (every six weeks) for natalizumab compared to standard interval dosing in healthy volunteerspatients with RMS and participants with Alzheimer’s disease.
In June 2017 we dosed ourenrolled the first patient in December 2018.
In December 2018 we dosed the first patient in a bioequivalence study to test whether exposure levels of PLEGRIDY are maintained with intramuscular administration.
Neuromuscular Disorders
| • | | In September 2018 we enrolled the first patient in the Phase 1 study evaluating BIIB078(IONIS-C9Rx), an antisense oligonucleotide (ASO) drug candidate, in adults with C9ORF72-associated ALS. |
| • | | In December 2018 we and our collaboration partner Ionis Pharmaceuticals, Inc. (Ionis) announced results from a positive interim analysis of the ongoing Phase 1 study of BIIB067 (IONIS-SOD1Rx), an investigational treatment for ALS with superoxide dismutase 1 (SOD1) mutations. The interim analysis showed that, over a three-month period, BIIB067 resulted in a statistically significant lowering of SOD1 protein levels in the cerebrospinal fluid and a numerical trend towards slowing of clinical decline as measured by the ALS Functional Rating Scale Revised, both compared to placebo. |
Alzheimer’s Disease and Dementia
In May 2018 we initiated a Phase 2 study of BIIB092 for Alzheimer’s disease.
In June 2018 we and our collaboration partner Eisai Co., Ltd. (Eisai) announced that elenbecestat, the oral BACE (beta amyloid cleaving enzyme) inhibitor, demonstrated an acceptable safety and tolerability profile in the Phase 2 study, and the results demonstrated a statistically significant difference in amyloid-beta levels in the brain measured byamyloid-PET (positron emission tomography). A numerical slowing of decline in functional clinical scales of a potentially clinically important difference was also observed, although this effect was not statistically significant.
In December 2017 we and our collaboration partner Eisai announced that the Phase 2 study of BAN2401, a monoclonal antibody targeting tau,that targets amyloid beta aggregates, an Eisai product candidate for PSP.
the treatment of Alzheimer’s disease, did not meet the criteria for success based on a Bayesian analysis at 12 months as the primary endpoint in an856-patient Phase 2 clinical study, an endpoint that was designed to enable a potentially more rapid entry into Phase 3 development. In July 20172018, based upon the final analysis of the data at 18 months, we and Eisai announced that the topline results from the Phase 2 study demonstrated a statistically significant slowing in clinical decline and reduction of amyloid beta accumulated in the brain. The study achieved statistical significance on key predefined endpoints evaluating efficacy at 18 months on slowing progression in Alzheimer’s Disease Composite Score (ADCOMS) and on reduction of amyloid accumulated in the brain as measured usingamyloid-PET.
In July 2018 we completed enrollment of ENGAGE and EMERGE, the Phase 3 studies of aducanumab. In March 2019 we and our collaboration partner Eisai announced that we were discontinuing the EMERGE and ENGAGE Phase 3 studies.
Movement Disorders
In January 2018 we dosed the first patient in the Phase 12 SPARK study of BIIB054,a-synuclein antibody, in both healthy volunteers and patients with early onset Parkinson’s disease.
In October 2017September 2018 we initiatedcompleted enrollment of the Phase 2b clinical trial AFFINITY, designed to evaluate opicinumab,anti-LINGO-1, as an investigationaladd-on therapy2 PASSPORT study of BIIB092 for PSP.
Acute Neurology
In March 2018 we dosed the first patient in people with relapsing MS.
In October 2017 we initiated the Phase 2 OPUS study evaluating the efficacy, safety and tolerability of natalizumaba4-integrin inhibitor, in drug-resistant focal epilepsy.
Business Development
In January 2017September 2018 we entered into a settlement and license agreement with Forward Pharma A/S (Forward Pharma). Pursuant to this agreement, we obtained U.S. and rest of world licenses to Forward Pharma’s intellectual property, including Forward Pharma’s intellectual property related to TECFIDERA.
In May 2017 we completed an asset purchase ofenrolled the first patient in the Phase3-ready candidate 3 CHARM study of BIIB093, (intravenous glibencamide) (formerly known as CIRARA) from Remedy Pharmaceuticals Inc. The target indication for BIIB093 isglibenclamide IV, in large hemispheric infarction, a severe form of ischemic stroke where brain swelling (cerebral edema) often leads to a disproportionately large share of stroke-related morbidity and mortality. The U.S. Food and Drug Administration (FDA) recently granted BIIB093 Orphan Drug Designation for severe cerebral edema in patients with acute ischemic stroke. The FDA has also granted BIIB093 Fast Track designation.
In June 2017 we completed an exclusive license agreement with Bristol-Myers Squibb Company for BIIB092 (formerly known asBMS-986168), a Phase2-ready experimental medicine with potential in Alzheimer’s disease and PSP. BIIB092 is an antibody targeting tau, the protein that forms the deposits, or tangles, in the brain associated with Alzheimer’s disease and other neurodegenerative tauopathies such as PSP.
In October 2017 we entered into a new collaboration agreement with Eisai Co. Ltd. (Eisai) for the joint development and commercialization of aducanumab, our anti-amyloid beta antibody candidate for Alzheimer’s disease. Under this agreement, we will continue to lead the ongoing Phase 3 development of aducanumab and will remain responsible for 100% of development costs for aducanumab until April 2018. Eisai will then reimburse us for 15% of aducanumab development expenses for the period April 2018 through December 2018, and 45% thereafter. Upon commercialization, both companies willco-promote aducanumab with a region-based profit split.
In October 2017 we amended the terms of our collaboration and license agreement with Neurimmune Subone AG (Neurimmune). Under the amended agreement, we made a $150.0 million payment to Neurimmune in exchange for a 15% reduction in royalty rates payable on products developed under the agreement, including on potential commercial sales of aducanumab. Our royalty rates payable on products developed under the agreement, including on potential commercial sales of aducanumab, will now range from the high single digits tolow-teens.
In November 2017 we entered into an exclusive license and collaboration agreement with Alkermes Pharma Ireland Limited, a subsidiary of Alkermes plc, for BIIB098 (formerly known as ALKS 8700), an oral monomethyl fumarate prodrug in Phase 3 development for the treatment of relapsing forms of MS.
In December 2017 we entered into a new collaboration agreement with Ionis to identify new antisense oligonucleotide (ASO) drug candidates for the treatment of SMA. Under this agreement, we have the option to license therapies arising out of this collaboration and will be responsible for the development and commercialization of these therapies.
Capital Allocation
In February 2017 we completed thespin-off of our hemophilia business, Bioverativ Inc., as an independent, publicly traded company.
Returned approximately $1.4 billion to stockholders in 2017 through share repurchases.
Announced a corporate restructuring program intended to streamline our operations and reallocate resources.
| | | | |
3133 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Neurocognitive Disorders
In December 2018 we dosed the first patient in our Phase 2b study of BIIB104 (AMPA) in CIAS.
Pain
In March 2018 we initiated a Phase 1 study of BIIB095, a Nav 1.7 inhibitor for neuropathic pain.
In May 2018 we initiated a Phase 2 study of vixotrigine (BIIB074) in small fiber neuropathy.
Other
In September 2018 we dosed the first patient in the Phase 2b study of BG00011(STX-100) in idiopathic pulmonary fibrosis, a chronic irreversible and ultimately fatal disease characterized by a progressive decline in lung function.
Discontinued Programs
In February 2018 we announced that the Phase 2b dose-ranging ACTION study investigating natalizumab in individuals with acute ischemic stroke (AIS) did not meet its primary endpoint. Based on these results, we discontinued development of natalizumab in AIS. The results of the Phase 2b ACTION study do not impact the benefit-risk profile of natalizumab in approved indications, including MS.
In October 2018 we announced that we completed the Phase 2b study of vixotrigine (BIIB074) for the treatment of painful lumbosacral radiculopathy (PLSR). The study did not meet its primary or secondary efficacy endpoints and we discontinued development of vixotrigine for the treatment of PLSR. The safety data were consistent with the safety profile reported in previous studies.
Business Development
In January 2018 we acquired BIIB100 from Karyopharm Therapeutics Inc. BIIB100 is a Phase 1 ready investigational oral compound for the treatment of certain neurological and neurodegenerative diseases, primarily in ALS. BIIB100 is a novel therapeutic candidate that works by inhibiting a protein known as XP01, with the goal of reducing inflammation and neurotoxicity, along with increasing neuroprotective responses.
In April 2018 we acquired BIIB104 from Pfizer Inc. BIIB104 is afirst-in-class, Phase 2b ready AMPA receptor potentiator for CIAS, representing our first program in neurocognitive disorders. AMPA receptors mediate fast excitatory synaptic transmission in the central nervous system, a process which can be disrupted in a number of neurological and psychiatric diseases, including schizophrenia.
In June 2018 we closed a10-year exclusive agreement with Ionis to develop novel ASO drug candidates for a broad range of neurological diseases (the 2018 Ionis Agreement). We have the option to license therapies arising out of the 2018 Ionis Agreement and will be responsible for the development and potential commercialization of such therapies.
In June 2018 we entered into an exclusive option agreement with TMS Co., Ltd. granting us the option to acquireTMS-007, a plasminogen activator with a novel mechanism of action associated with breaking down blood clots, which is in Phase 2 development in Japan, and backup compounds for the treatment of stroke.
In June 2018 we exercised our option under our joint venture agreement with Samsung BioLogics to increase our ownership percentage in Samsung Bioepis from approximately 5% to approximately 49.9%. The share purchase transaction was completed in November 2018.
In July 2018 we acquired BIIB110 (Phase 1a) andALG-802 (preclinical) from AliveGen Inc. BIIB110 andALG-802 represent novel ways of targeting the myostatin pathway. We initially plan to study BIIB110 in multiple neuromuscular indications, including SMA and ALS.
In December 2018 we exercised our option with Ionis and obtained a worldwide, exclusive, royalty-bearing license to develop and commercialize BIIB067, an investigational treatment for ALS with SOD1 mutations.
In December 2018 we entered into a collaborative research and license agreement with C4 Therapeutics (C4T) to investigate the use of C4T’s novel protein degradation platform to discover and develop potential new treatments for neurological diseases, such as Alzheimer’s disease and Parkinson’s disease. We will be responsible for the development and potential commercialization of any therapies resulting from this collaboration.
| | | | |
| 34 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Leadership TeamShare Repurchase Activity
At the core
In August 2018 our Board of what we do areDirectors authorized a program to repurchase up to $3.5 billion of our peoplecommon stock (2018 Share Repurchase Program). Our 2018 Share Repurchase Program does not have an expiration date. All share repurchases under our 2018 Share Repurchase Program will be retired.
We returned approximately $4.4 billion to stockholders in 2018 through share repurchases under our 2018 Share Repurchase Program and our leaders. As2016 Share Repurchase Program, which was a result,program authorized by our goal isBoard of Directors in July 2016 to findtop-tier talent with the skills necessaryrepurchase up to imagine$5.0 billion of our common stock and lead us into the future. We advanced this goal in 2017 by appointing several new executives in key roles. These appointments included:which was completed as of June 30, 2018.
• | | Michel Vounatsos, Chief Executive Officer, formerly Executive Vice President, Chief Commercial Officer.Mr. Vounatsos joined us in April 2016 as our Executive Vice President, Chief Commercial Officer after a20-year career with Merck and became our Chief Executive Officer in January 2017. While at Merck, he held leadership positions of increasing responsibility in Europe, China and the U.S., driving significant and consistent growth across multiple geographies. We believe that his significant knowledge and experience with respect to the biotechnology, healthcare and pharmaceutical industries, and his comprehensive leadership background, will guide Biogen in the next phase of its evolution. |
• | | Jeffrey D. Capello, Executive Vice President and Chief Financial Officer.Mr. Capello joined us in December 2017 as our Executive Vice President and Chief Financial Officer, bringing 26 years of experience in finance. Most recently he was Executive Vice President and Chief Financial Officer of Beacon Health Options Inc. His previous experience includes founding and running his own company, Monomy Advisors, and serving as Chief Financial Officer of Ortho Clinical Diagnostics, Boston Scientific Corporation and PerkinElmer. Earlier in his career he was a partner in the Boston and Amsterdam offices of PwC. We believe that Mr. Capello’s strong public company financial experience will allow him to play a critical role as we aim to execute on our business strategy, pursue business development opportunities and build our pipeline. |
• | | Ginger Gregory, Executive Vice President and Chief Human Resources Officer.Dr. Gregory joined us as our Executive Vice President, Chief Human Resources Officer in July 2017, bringing over 20 years of human resources experience to Biogen. She was most recently the Chief Human Resources Officer at Shire Pharmaceuticals. Prior to that, Dr. Gregory held executive-level human resources positions for several multinational companies across a variety of industries, including Dunkin’ Brands, where she served as Chief Human Resource Officer; Novartis, AG, where she was the division head of Human Resources for Novartis Vaccines and Diagnostics, Novartis Consumer Health and Novartis Institutes of BioMedical Research; and Novo Nordisk, where she served as Senior Vice President, Corporate People & Organization at the company’s headquarters in Copenhagen, Denmark. We believe that Dr. Gregory’s extensive experience in the biotechnology and pharmaceutical industries will be valuable as we endeavor to attract, develop and retain a talented, culturally diverse workforce to execute on our mission to transform neuroscience and the treatment of neurological diseases. |
• | | Chirfi Guindo, Executive Vice President and Head of Global Marketing, Market Access and Customer Innovation.Mr. Guindo joined us as our Executive Vice President and Head of Global Marketing, Market Access and Customer Innovation in November 2017. Mr. Guindo brings 27 years of experience in the global pharmaceutical industry and has held several leadership positions at Merck (known as MSD outside Canada and the U.S.) in Canada, the U.S., France, Africa and the Netherlands. He has worked in several disciplines including Finance, Sales & Marketing, General Management and Global Strategy/Product Development in specialty, acute and hospital care. Most recently Mr. Guindo was President & Managing Director of Merck Canada. We believe that Mr. Guindo’s extensive experience in the global pharmaceutical industry will help us further our leadership in MS and SMA, plan for our Alzheimer’s disease franchise and pursue future pipeline opportunities. |
Other Notable Achievements in the Workplace and Community
Awarded with our collaboration partner Ionis, the 20172018 International Prix Galien USA Award foras Best Biotechnology Product for SPINRAZA. The prestigious honor marks the seventh Prix Galien for SPINRAZA, following country recognitions in the U.S., Germany, Italy, Belgium-Luxembourg, the Netherlands and the U.K. The International Prix Galien is given every two years by Prix Galien International Committee members in recognition of excellence in scientific innovation to improve human health.
Named the Biotechnology Industry Leader on the Dow Jones Sustainability World Index.
Recognized as a corporate sustainability leader with Gold Class and Industry Mover Sustainability Awards from RobecoSAM.
Continued commitment to operational carbon neutrality highlighted bythrough the use of 100% renewable electricity globally.
Committed to reduce carbon emissions by a targeted amount approved by the Science Based Target Initiative, to align ourselves with the global goal of limiting global temperature rise to under two degrees Celsius.
Recognized as a corporate sustainability leader with naming toEarned CDP scores of A,A- and B in the CDP A List for bothareas of Supplier Engagement, Climate Change and Water, and receiving RobecoSAM Silver Class and Industry Mover distinctions.respectively.
Earned a perfect score of 100% on the Human Rights Campaign’s Corporate Equality Index (a national benchmarking tool on corporate policies and practices pertinent to LGBTQ employees) for the fourthfifth consecutive year.
Continued commitment to diversity and inclusion. As of December 31, 2018, 44% of Director-level positions and above were held by women.
Over 2,6003,200 employees volunteered from 2628 countries induring our annual Care Deeply Day.
Engaged 44,000+50,000+ students inhands-on learning to inspire their passion for science since the inception of Biogen’s Community Labs.
| | | | |
32 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
Total Stockholder Return
Ourone-, three- and five-year total stockholder return (TSR)* compared to our peer group and the Standard & Poor’s 500 (S&P 500) is set forth below.
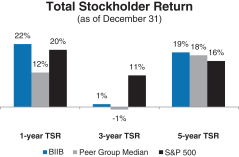
* | TSR is a measure of performance over time that combines changes in share price and dividends paid to show the total return to the stockholder expressed as an annualized percentage. |
20172018 Executive Compensation Programs andPay-for-Performance Alignment
We believe our executive compensation programs are effectively designed and have worked well to implement apay-for-performance culture that is aligned with the interests of our stockholders. In 20172018 our executive compensation programs consisted of base salary, short- and long-term incentives and other benefits.
91% of our CEO’s and 84% of our other current NEOs’ 20172018 target compensation was performance-based andat-risk.
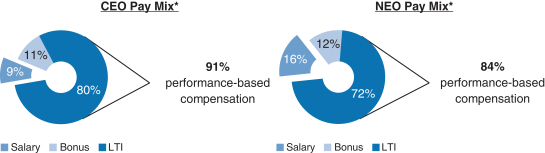
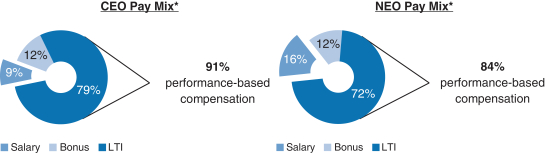
| | * | Reflects annual salary, target bonus and target grant date value of the 20172018 annual long-term incentive awards. The CEO compensation mix reflects compensation for Mr. Vounatsos, who has served as our CEO since January 6, 2017. The NEO compensation mix excludes Dr. McKenzie’stheone-time RSU award,transition awards of RSUs granted to Dr. Ehlers, Ms. Alexander and Dr. McKenzie, as described in further detail below, as well as compensation for Dr. Scangos and Messrs. Capello, Clancy and DiPietro due to partial year employment with Biogen in 2017 and compensation for Mr. Covino, due to his change in roles during 2017.below. |
| | | | |
3335 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
100% of our NEOs’ 2018 annual long-term incentive (LTI) grants were performance-based andat-risk.
| | |
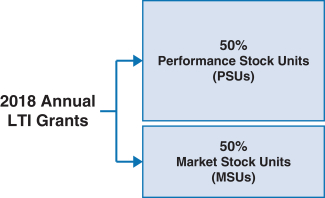 | | • 60% earned based on achievement of three-year adjustedNon-GAAP diluted earnings per share (EPS) and pipeline milestone performance goals • 40% earned based on achievement of adjustedNon-GAAP free cash flows and revenues over threeone-year performance periods • PSUs were introduced in 2018. For more information on our PSUs, please see “Long-Term Incentives – 2018 PSUs” below. • Earned based on stock price performance over one, two and three year periods |
Our 20172018 performance-based compensation payouts align with our commitment to strong performance.
In 2017 overall2018 we achieved or exceeded the vast majority of the corporate performance goals that we set at the beginning of the year for our incentive compensation plans. As a result, the payouts, as a percentage of target, for our 20172018 annual bonus plan 2017 awarded cash-settledand the portions of our PSUs and MSUs that were eligible to be earned based on 2018 performance units (CSPUs) and 2017 awarded market stock units (MSUs) were above target payout amounts, as described in further detail below.
| | | | |
Annual Bonus Plan
130%*
Company Performance Multiplier
(The overall annual bonus plan multiplier for each NEO was further modified based on his/her individual performance multiplier)
| | Cash-Settled Performance Units
123%*
Performance multiplier for the CSPUs
during the 2017 performance period
(Earned units are subject to three-year
time vesting from the grant date)
| | Market Stock Units
126%*
Performance multiplier for the MSUs during the 2017 performance period
|
* | Actual multiplier for applicable 2017 award based on corporate performance. |
20172018 Advisory Vote on Executive Compensation
| | |
| At our 2018 annual meeting of stockholders, we continued to receive strong support for our executive compensation programs with approximately 95% of the votes cast for approval of our annual“say-on-pay” proposal. Our C&MD Committee viewed this as positive support for our executive compensation programs and their alignment with long-term stockholder value creation and determined that the Company’s executive compensation programs have been effective in implementing the Company’s stated compensation philosophy and objectives. | | 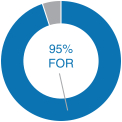
|
Our C&MD Committee is committed to continually reviewing our executive compensation programs on a proactive basis to ensure the ongoing alignment of such programs with the interests of our stockholders. |
| In 2018 our C&MD Committee reviewed the external landscape, the results from our“say-on-pay” proposal at last year’s annual meeting of stockholders and the Company’s performance against the current compensation programs. Our C&MD Committee was satisfied that our existing compensation programs further ourpay-for-performance philosophy, but made certain enhancements to the design of our LTI program in 2018 to strengthen its focus on long-term performance and alignment with our stockholders’ interests. |
Specifically, under our 20172018 LTI program, grants of PSUs replaced grants of cash-settled performance units (CSPUs), which we had granted in previous years. The key changes are as follows:
PSU awards are subject to three-year cliff vesting as compared to annual meetingratable vesting over three years (1/3 per year) for CSPU awards;
60% of stockholders, we continuedPSU awards are earned over a three-year performance period based on the achievement of three-year cumulative performance goals for stock-settled PSU awards and 40% of PSU awards are earned over three annual performance periods based on the achievement of three sets of annual performance goals for cash-settled PSU awards as compared to receive support100% of CSPUs awards earned based upon one annual performance period for our executive compensation programs with approximately 97%CSPU awards; and
60% of the votes castPSU awards will be settled in stock and 40% of the PSU awards will be settled in cash as compared to 100% cash settlement for approval of our annual“say-on-pay” proposal. Our Compensation Committee viewed this as very positive support for our executive compensation programs and their alignment with long-term stockholder value creation and noted that the Company’s executive compensation programs have been effective in implementing the Company’s stated compensation philosophy and objectives.CSPU awards.
Our Compensation Committee is committed to continually reviewing our executive compensation programs on a proactive basis to ensure the ongoing alignment of such programs with the interests of our stockholders.
In 2017 we reviewed the external landscape, the results from our“say-on-pay” proposal at last year’s annual meeting of stockholders and the results of our current compensation programs. Our Compensation Committee was satisfied that our existing compensation programs further ourpay-for-performance philosophy, and, accordingly, did not recommend any significant changes to our executive compensation programs for 2017.
| | | | |
3436 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
For additional information on our PSU awards, please see “Long-Term Incentives – 2018 PSUs” below.
Roles and Responsibilities
Role of our CompensationC&MD Committee
Our CompensationC&MD Committee, which is composed of four independent directors, oversees and administers our executive compensation programs. In making executive compensation decisions, our CompensationC&MD Committee considersreviews a variety of factors and data, most importantly our performance and individual executives’ performance, and takes into accountconsiders the totality of compensation that may be paid. In addition, our CompensationC&MD Committee administers our annual bonus plan and our equity plans, reviews business achievements relevant to payouts under our compensation levels,plans, makes recommendations to our Board of Directors with respect to compensation policies and practices as well as the compensation of our CEO and seeks to ensure that total compensation paid to our executive officers is fair, competitive and aligned with stockholder interests. Our CompensationC&MD Committee retains the right to hire outside advisors as needed to assist it in reviewing and revising our executive compensation programs.
The duties and responsibilities of our CompensationC&MD Committee are described on page 1920 and can be found in our CompensationC&MD Committee’s written charter adopted by our Board of Directors, which can be found on our website,www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of the website.
Role of the Independent Compensation Consultant
Our CompensationC&MD Committee believes that independent advice is important in developing Biogen’sand overseeing our executive compensation programs. Frederic W. Cook & Co., Inc. (FW Cook) is currently engagedserved as our CompensationC&MD Committee’s independent compensation consultant.consultant until June 2018 and advised our C&MD Committee regarding compensation decisions in 2018. FW Cook did not provide any other services to Biogen. Pearl Meyer & Partners LLC (Pearl Meyer) has served as our C&MD Committee’s independent compensation consultant since June 2018 and has advised our C&MD Committee regarding compensation decisions since that time. Pearl Meyer does not provide any other services to Biogen.Biogen and engages in other matters as needed and as directed solely by our C&MD Committee. References in this CD&A to our independent compensation consultant refer to FW Cook for the period during which it was engaged and to Pearl Meyer thereafter.
Reporting directly to our CompensationC&MD Committee, FW Cookour independent compensation consultant provides guidance on trends in CEO, executive andnon-employee director compensation, the development of specific executive compensation programs and the composition of the Company’s compensation peer group. Additionally, FW Cookour independent compensation consultant prepares a report on CEO pay that compares each element of compensation to that of CEOs in comparable positions at companies in our peer group. Using this and other similar information, our CompensationC&MD Committee recommends, and our Board of Directors approves, the elements and target levels of our CEO’s compensation. FW Cook also engages in other matters as needed and as directed solely by our Compensation Committee.
During 20172018 the Company paid FW Cook $250,989and Pearl Meyer $123,275 and $47,666, respectively, in consulting fees directly related to these services. Our CompensationC&MD Committee assessesassessed FW Cook’s independence annually and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2017 that FW Cook’s work did not raise any conflicts of interest and that FW Cook remained independent under applicable rules. Our C&MD Committee assessed Pearl Meyer’s independence in connection with its engagement in June 2018 and, in accordance with applicable SEC and Nasdaq rules, confirmed in December 2018 that Pearl Meyer’s work did not raise any conflicts of interest and that Pearl Meyer remains independent under applicable rules.
Role of our CEO
Each year our CEO provides an assessment of the performance of each executive officer, other than himself, during the prior year and recommends to our CompensationC&MD Committee the compensation to be paid or awarded to each executive. Our CEO’s recommendations are based on numerous factors, including:
Company, team and individual performance;
potential for future contributions;
leadership competencies;
external market competitiveness;
internal pay comparisons; and
other factors deemed relevant.
To understand the external market competitiveness of the compensation for our executive officers, our CEO and our CompensationC&MD Committee review a report analyzing publicly-available information and surveys prepared by our internal
| | | | |
| 37 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
compensation group and reviewed by FW Cook.our independent compensation consultant. The report compares the compensation of each executive officer, other than our CEO, to data for comparable positions at companies in our peer group, by compensation element (see(please see “External Market Competitiveness and Peer Group” below for further details). Our CompensationC&MD Committee considers all of the information presented, discusses the recommendations with our CEO and with FW Cookour independent compensation consultant and applies its judgment to determine the elements of compensation and target compensation levels for each executive officer other than the CEO.
Our CEO also provides a self-assessment of his achievements for the prior year. Our CompensationC&MD Committee reviews and considers this in analyzing the CEO’s performance, and in recommending for approval by our Board of Directors, the compensation of our CEO. Our CEO does not participate in any deliberations regarding his own compensation.
Executive Compensation Philosophy and Objectives
Our executive compensation programs are designed to drive the creation of long-term stockholder value by deliver-
| | | | |
35 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
ingdelivering performance-based compensation that is competitive with our peer group in order to attract and retain extraordinary leaders who can perform at high levels and succeed in a demanding business environment. We aim to achieve this by designing programs that are:
| • | | Mission Focused and Business Driven. Our executive compensation programs support the relentless pursuit of delivering meaningful and innovative therapies to patients by providing our executives with incentives to achieve the near- and long-term objectives of our business. Substantially all of our executive incentive compensation programs are tied directly, and meaningfully, to Company performance. Our objective is to emphasize the importance of achieving short-term goals while building and sustaining a foundation for long-term success. |
| • | | Competitively Advantageous. We benchmark our executive compensation programs against a peer group of biotechnology and pharmaceutical companies that we believe are representative of the companies we primarily compete with for talent, balanced with factors such as business scope and size, including revenues and market capitalization, business focus and geographic scope of operations. We consider peerPeer group practices as one ofare among the many factors to be takenwe take into account in developing compensation programs that we believe are most meaningful to our leaderseffective, and the Company, and which |
| | enable us to recruit, retain and motivate our leadership team to achieve their best for Biogen and our stockholders. |
| • | | Performance Differentiated. We believe strongly inpay-for-performance and endeavor to significantly differentiate rewards by delivering the highest rewards to our best performers and little or nolesser rewards to those who do not perform atpre-established levels.meet our performance expectations. |
| • | | Ownership Aligned. At Biogen, we believe every employee contributes to the success of the Company and, as such, every employee has a vested interest in the Company’s success. To reinforce this alignment with our stockholders, we strongly encourage stock ownership through our equity-based compensation programs. For members of our executive team, including our NEOs, who set and lead the future strategic direction of our Company, we ensure that a significant portion of their total pay opportunities are equity-based to maintain alignment between the interests of our executive officers and our stockholders. |
| • | | Flexible. We are committed to providing flexible benefits designed to allow our diverse global workforce to have reward opportunities that meet their varied needs so that they are inspired to perform their very best on behalf of patients and stockholders each day. |
External Market Competitiveness and Peer Group
MarketWe consider market practices are one of the considerations taken into accountand trends when determining executive compensation levels and compensation program designs at Biogen. We do not target a specific market percentile or simply replicate the market practice. Instead, we review external market practices as a reference point to assist us in providing programs designed to attract, retain and inspire extraordinary talent. Our CompensationC&MD Committee also uses a peer group to provide context for its executive compensation decision-making. Each year our independent compensation consultant reviews the external market landscape and evaluates the composition of our peer group for appropriateness.
Our CompensationC&MD Committee reviews the information provided from internal sources as well as the information provided by our independent compensation consultant to select our peer group based on comparable companies that approximate (1) our scope of business, including revenues and market capitalization, (2) our global geographical reach, (3) our research-based business with multiple marketed products and (4) a comparable pool of talent for which we compete.
The peer group for determining our February 20172018 compensation decisions consisted of biotechnology and pharmaceutical
| | | | |
| 38 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
companies, as we compete with companies in both of these sectors for executive talent.
|
|
| Biotechnology Peers |
Alexion Pharmaceuticals, Inc. Amgen Inc. Celgene Corporation Gilead Sciences Inc. Vertex Pharmaceuticals International, Inc. |
|
| Pharmaceutical Peers |
AbbVie Inc. Allergan plc Bristol-Myers Squibb Company Eli Lilly and Company Endo Health Solutions Merck & Co, Inc. Mylan N.V. Bausch Health Companies (f/k/a Valeant Pharmaceuticals IncorporatedIncorporated) |
For each of the companies in our peer group, where available, we analyze the company’s Compensation Discussion and Analysis and other data publicly filed during the prior year to identify the executives at such companies whose positions are comparable to those held by our executive officers. We then compile and analyze the data for each
| | | | |
36 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
comparable position. Our competitive analysis includes the structure and design of the compensation programs as well as the targeted value of the compensation under these programs.
For our executive officers other than our CEO, we may supplement the data forfrom our peer group with published compensation surveys where appropriate. For 2017,2018, consistent with past years, we used theWillisTowersWatson U.S. CDB Pharmaceutical and Health Sciences Executive Compensation Database survey (which we refer to as the Willis Towers Watson survey). We chose thisthe Willis Towers Watson survey because of the number of companies in our peer group that participate in it, the number of positions reported by the survey that continue to be comparable to our executive positions and the high standards under which we understand the survey is conducted (including data collection and analysis methodologies). All of the companies in our peer group are represented in a special cross-section of the Willis Towers Watson survey focused on our peer group, other than Bausch Health Companies (formally known as Valeant Pharmaceuticals Incorporated,Incorporated), which did not participate in the survey.
Compensation Elements
Our CompensationC&MD Committee determines the elements of compensation we provide to our executive officers. The elements of
our executive compensation programs and their objectives are as follows:
| | | | | | |
| | | |
| Element | | | | Objective(s) | | |
Base Salary | | • | | Provides a fixed level of compensation that is competitive with the external market and reflects each executive’s contributions, experience, responsibilities and potential to contribute to our future success. | | |
Annual Bonus Plan | | • | | Aligns short-term compensation with the annual goals of the Company. | | |
| | • | | Motivates and rewards the achievement of annual Company and individual performance goals that support short- and long-term value creation. | | |
Long-term Incentives | | • | | Aligns executives’ interests with the long-term interests of our stockholders by linking the value of awards to increases in our stock price. | | |
| | • | | Motivates and rewards the achievement of stock price growth andpre-established corporate performance goals.goals, including those with a longer-term focus. | | |
| | • | | Promotes executive retention and stock ownership and focuses executives on enhancing long-term stockholder value. | | |
Benefits | | • | | Promotes health and wellness. | | |
| | • | | Provides financial protection in the event of disability or death. | | |
| | | • | | Providestax-beneficial ways for executives to save towards their retirement and encourages savings through competitive matches to executives’ retirement savings. | | |
Compensation Mix
Our CompensationC&MD Committee determines the general mix of the elements of our executive compensation programs. It does not target a specific mix of value for the compensation elements within these programs in either the program design or pay decisions. Rather, our CompensationC&MD Committee reviews the mix of compensation mixelements to ensure an appropriate level of performance-based compensation is apportioned to the short-term and even more to the long-term to ensure alignment with our business goals and performance.
Additionally, our CompensationC&MD Committee believes the greater the leadership responsibilities, the greater the potential impact an individual will have on Biogen’s future strategic direction. Therefore, for our executive officers, including our NEOs, additional emphasis is placed on performance-based compensation, with a particular emphasis on long-term incentives (LTI).LTI.
The 20172018 compensation mix for Mr. Vounatsos and our other NEOs was highly performance-based andat-risk; 91% of 20172018 compensation was performance-based for Mr. Vounatsos and 84% of 20172018 compensation was
| | | | |
| 39 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
performance-based for our other current NEOs, (other than Messrs. Capello and Covino), assuming target level achievement of applicable corporate performance goals and with LTI awards measured at target grant date
values, and excluding Dr. McKenzie’stheone-time RSU award,transition awards of RSUs granted to Dr. Ehlers, Ms. Alexander and Dr. McKenzie, as described in further detail below.
Performance Goals and Target Setting Process
Early each year, our C&MD Committee reviews and establishes the pay levels of each element of total compensation for our executive officers. Total compensation is comprised of base salary, annual bonus and LTI awards.
As part of this process, our C&MD Committee reviews the mix of compensation elements to ensure our performance-based compensation is apportioned appropriately and aligns with our business goals and performance. Our C&MD Committee also ensures that the performance metrics and goals are aligned with the annual business plan approved by our Board of Directors so there is full alignment of executive incentive goals with the goals that have been established for the year. Executive officers are also evaluated based on qualitative factors, such as individual, strategic and leadership achievements. The use of both quantitative and qualitative metrics, as well as the weighting of such metrics, effectively mitigates the impact of a single risk, such as dependence on drug pricing, pipeline performance or market share, on overall compensation.
| | | | |
3740 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Performance Goals and Target Setting Process
Early each year, our Compensation Committee reviews and establishes the pay levels of each element of total compensation for our executive officers. Total compensation is comprised of base salary, annual bonus and LTI awards. A summary of the process our CompensationC&MD Committee follows in setting compensation is described below:
| | | | |
  Target Setting Target Setting
| |  
  Monitoring & Tracking Monitoring & Tracking
• Our CompensationC&MD Committee closely monitors the progress against the performance goals throughout the year and engages in dialogue with management on such progress. | |   Results & Awards: Results & Awards:
CompensationC&MD Committee Actions
• Reviews and certifies the annual Company results against thepre-established goals for our incentive compensation plans.
• Reviews and discusses the performance of our executive officers against their respective performance goals, including our CEO.goals. • Reviews and discusses the Company, team and individual performance of each executive officer, other than our CEO, as assessed by our CEO. • Reviews and discusses our CEO’s recommended compensation levels for each executive officer, other than himself, in the context of such executive officer’s contributions to the Company and the other factors described above. • Approves the final compensation for each NEOexecutive officer other than our CEO, including base salary, annual bonus and LTI awards. • Reviews CEO compensation and recommends to our Board of Directors for approval the compensation of our CEO, including base salary, annual bonus and LTI awards. |
• Our Compensation Committee and our CEO discuss potential goals for the upcoming year that are tied to the short- and longer-term strategic goals of the Company. • Our CompensationC&MD Committee and our CEO discuss potential goals for the upcoming year that are tied to the short- and longer-term strategic goals of the Company as well as individual goals for our executive officers.
• The annual business plan for the year is approved by our Board of Directors,Directors. As part of the approval process, our Board considers many factors relevant to our business, reputation and strategy, including pipeline and business development, pricing and patient access, market expectations and intellectual property risk. • Our C&MD Committee ensures that the performance goals and targets under our compensation plans are aligned with the approved annual business plan. • Payout levels for each performance goal are established by management and approved by our CompensationC&MD Committee. • The performance goals are then applied to the compensation opportunities for our executive officers, including NEOs, so that there is full alignment of executive incentive goals with the goals that have been established for the year. • Our CompensationC&MD Committee also reviews base salaries, bonus and LTI planning ranges, plan designs, benefits and peer group data. | | |
| | | | |
3841 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
2017 and 2018 Hiring- and Transition-Related Compensation Decisions
Arrangement with Mr. Vounatsos
In connection with Mr. Vounatsos’ appointment as our Chief Executive Officer effective as of January 6, 2017, our Compensation Committee approved, in December 2016, as part of the employment agreement he entered into with us, an increase in his annual base salary to $1.1 million and a target bonus of 125% of his annual base salary under our annual bonus plan, in each case, effective upon his appointment as our Chief Executive Officer on January 6, 2017. In addition, on February 15, 2017, he received a LTI award of CSPUs with a grant date fair value of $4,999,754 and a LTI award of MSUs with a grant date fair value of $4,868,786. The terms of the CSPUs and MSUs granted to Mr. Vounatsos are described below under the heading “Long-Term Incentives (LTI)” and the payments that Mr. Vounatsos would be eligible to receive under his employment agreement in connection with certain terminations of employment are described in further detail under the heading “Potential Payments Upon Termination or Change in Control” below. Our Compensation Committee approved these terms after reviewing peer group data provided by FW Cook.
Arrangement with Mr. Capello
In November 2017 we appointed Mr. Capello as our Executive Vice President and Chief Financial Officer, effective as of December 11, 2017.
In determining the annual and long-term compensation for Mr. Capello, our Compensation Committee followed the same compensation philosophy and objectives described in this CD&A and also took into consideration the value of compensation that Mr. Capello would have been eligible to earn had he remained employed by his prior employer. After considering the compensation opportunities that Mr. Capello would be required to forfeit in order to join us, and in order to incentivize him to do so, our Compensation Committee granted Mr. Capello aone-time cashsign-on bonus of $520,000. Our Compensation Committee also approved an annual base salary for Mr. Capello of $750,000 and, beginning in 2018, a target bonus of 70% of his annual base salary under our annual bonus plan.
Mr. Capello’sone-time cashsign-on bonus is subject to repayment to the Company in the event Mr. Capello voluntarily terminates his employment or his employment is terminated by us for cause (as defined in our 2017 Omnibus Equity Plan) or for misconduct or poor performance, as determined by us in good faith, as follows: 100% of his cashsign-on bonus is subject to repayment if such termination
occurs within the first year of his employment, 70% of his cashsign-on bonus is subject to repayment if such termination occurs within the second year of his employment and 35% of his cashsign-on bonus is subject to repayment if such termination occurs within the third year of his employment, in each case, net of applicable tax withholdings.
In connection with Mr. Capello’s appointment, our Compensation Committee also granted him a LTI award in January 2018, which consisted of performance stock units (PSUs) and MSUs with an aggregate grant date target value of $3.0 million. The terms of the PSUs and MSUs awards granted to Mr. Capello are described below under the heading “Long-Term Incentives (LTI).” Because of this grant, Mr. Capello was not eligible to receive an annual LTI award in 2018.
Arrangement with Dr. McKenzie
In February 2016 Dr. McKenzie was appointed as our Senior Vice President for Global Biologics Manufacturing & Technical Operations. As part of his appointment, Dr. McKenzie was eligible to earn aone-time LTI award of RSUs that were contingent upon his achievement of at least a “solid” performance rating for 2016. This RSU award was intended to represent a portion of the compensation that Dr. McKenzie would have been eligible to receive had he remained with his prior employer. In March 2017, based upon his 2016 performance rating, Dr. McKenzie was granted RSUs with a grant date fair value of $800,600. These RSUs vest in three equal annual installments beginning on the first anniversary of the date of grant, subject to Dr. McKenzie’s continued employment.
Dr. Scangos’ Arrangements
On January 6, 2017, Dr. Scangos ceased to be our Chief Executive Officer (which was considered a termination without cause under his employment agreement) and the Company paid him the severance benefits payable under his employment agreement, consisting of a lump sum cash payment in the amount of $7.2 million (two times his annual base salary and target annual bonus), a prorated bonus payment of $44,877 for the period January 1, 2017 through January 6, 2017, which was based on actual Company performance and deemed 100% individual performance (as provided under his employment agreement) and continuation of certain subsidized medical, dental and vision benefits until July 1, 2018. Dr. Scangos was also entitled to receive up to nine months of executive-level outplacement services at our cost; however, Dr. Scangos did not utilize these services. In addition, pursuant to the terms of his
| | | | |
39 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
employment agreement, all of his outstanding MSUs, CSPUs and stock options continue to vest as if he had remained employed by the Company for the duration of the respective award’s vesting period and all awards that require exercise by him remain exercisable until the earlier of January 7, 2020 or their respective expiration date.
Mr. Clancy’s Arrangements
Mr. Clancy voluntarily separated from the Company on July 1, 2017 and did not receive any severance benefits in connection with his separation. Mr. Clancy’s outstanding LTI awards were eligible for retirement vesting with either accelerated or continued vesting, as applicable, pursuant to the retirement provision under our 2008 Omnibus Equity Plan.
Mr. DiPietro’s Arrangements
On May 26, 2017, Mr. DiPietro ceased to be our Executive Vice President, Human Resources and ceased to be employed by us effective September 30, 2017. We provided him the severance benefits required under our executive severance policy for Executive Vice Presidents, which consisted of a lump sum payment of $2,019,413 (21 months of base salary and target bonus) and continuation of certain subsidized medical, dental and vision benefits until the earlier of (1) January 31, 2019 or (2) the date on which he becomes eligible to receive benefits through another employer. In addition, our Compensation Committee agreed to permit the continued vesting ofone-third of his outstanding LTI awards scheduled to vest on February 15, 2018, February 22, 2018, and February 23, 2018. Mr. DiPietro was also eligible to receive up to 12 months of executive-level outplacement services at our cost; however, Mr. DiPietro did not utilize these services.
2017 Base Salary
In determining Mr. Vounatsos’ base salary as our CEO, ourOur Board of Directors reviewed the base salaries of comparable chief executive officers in our peer group and considered Mr. Vounatsos’ compensation mix, capabilities, performance and future expected contributions. Based on its review, Mr. Vounatsos’ base salary was set at $1,100,000,$1,300,000, which positioned him below the 25th percentilemarket median when compared to the chief executive officers of our peer group.
Our CompensationC&MD Committee undertook a similar review when approving the base salaries for our other NEOs, which positioned them, on average, slightly below the market median compared to persons with comparable jobs within our peer group.
The annual base salary of each of our NEOs in 20172018, compared to 20162017, was as follows:
| Name | | 2016 Salary | | | 2017 Salary | | | % Increase(1) | | | 2018 Salary | | | 2017 Salary | | | % Increase(1) | |
M. Vounatsos | | $ | 750,000 | | | $ | 1,100,000 | | | 46.7% | | | | $ | 1,300,000 | | | $ | 1,100,000 | | | 18.2% | | |
J. Capello(2) | | | n/a | | | $ | 750,000 | | | n/a | | | | $ | 750,000 | | | $ | 750,000 | | | n/a | | |
M. Ehlers | | $ | 775,000 | | | $ | 794,375 | | | 2.5% | | | | $ | 834,094 | | | $ | 794,375 | | | 5.0% | | |
S. Alexander | | $ | 696,002 | | | $ | 723,842 | | | 4.0% | | | | $ | 749,177 | | | $ | 723,842 | | | 3.5% | | |
P. McKenzie | | $ | 575,000 | | | $ | 603,750 | | | 5.0% | | | | $ | 633,938 | | | $ | 603,750 | | | 5.0% | | |
G. Covino | | $ | 375,315 | | | $ | 386,574 | | | 3.0% | | | |
G. Scangos(3) | | $ | 1,500,000 | | | $ | 1,500,000 | | | — | | | |
P. Clancy | | $ | 860,470 | | | $ | 890,586 | | | 3.5% | | | |
K. DiPietro | | $ | 655,840 | | | $ | 678,794 | | | 3.5% | | | |
| (1) | Percentage increase reflects the annual merit increase for all NEOs other than for Mr. Vounatsos and, in the case of Mr. Vounatsos, represents an increase asalso includes a result of his appointment as our CEO.market adjustment. |
| (2) | Mr. Capello was hired in November 2017. The initial determination of his base salary took into account the Company’s peer group data. |
(3) | Due to the fact that Dr. Scangos’ employment terminated in January 2017 his base salary was not considered for an increase for 2017. |
20172018 Performance-Based Plans and Goal Setting
Our executive compensation programs place a heavy emphasis on performance-based compensation.
We maintain a short-term incentive plan, known as our annual bonus plan, as well as aan LTI plan.
Awards to our NEOs under our annual bonus plan arehave been made under our 2008 Performance-Based Management Incentive Plan, and awards under our LTI plan are granted under our 2017 Omnibus Equity Plan.
Awards made under our annual bonus plan are directly tied to the achievement of our corporate performance goals, which are aligned with the Company’s short- and long-term strategic plans, as well as individual performance goals.
Awards made under our LTI plan are directly tied to the performance of the price of our common stock, which alignaligns our executives’ long-term interests with the interests of our stockholders. SomeA portion of our LTIsLTI awards are also tied to the Company’s financial performance, as described below under “2017 CSPU Company Performance Targets and Results Table.“Long-Term Incentives – 2018 PSUs.”
In setting our annual goals under our short- and long-term incentive plans, in addition to our internal forecasts, we consider analysts’ projections for our performance and the performance of companies in our peer group, as well as broad economic and industry trends. We strive to establish challenging targets that result in payouts at or above target
| | | | |
40 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
levels only when Company performance warrants it. Our CompensationC&MD Committee is responsible for reviewing and approving our annual goals, targets and levels of payout (e.g., threshold, target and maximum) for our executive incentive compensation plans and for reviewing and determining actual performance results at the end of the applicable performance period.
In setting and approving the corporate performance goals for our executive officers and for the Company under both the short- and long-term incentive plans, our CompensationC&MD Committee also considers the alignment of such goals to our business plan, the degree of difficulty of attainment and the potential for the goals to encourage inappropriate risk-taking. Our CompensationC&MD Committee has determined that the structures of our executive compensation programs do not put our patients, investors or the Company at any material risk.
Annual Bonus Plan
Our annual bonus plan is a cash incentive plan that rewards near-term financial, strategic and operational performance. Our CompensationC&MD Committee reviews ourthe annual target bonus opportunities for each executive officer by job levelposition each year to ensure such opportunities areremain competitive.
No significant changes were made in 20172018 to the target annual bonus opportunities, as a percentage ofyear-end annual base salary, for any of our NEOs other than Mr. Vounatsos, whose target annual bonus opportunity was market adjusted and increased from 125% of base salary in connection with his appointment as our CEO2017 to 140% of base salary in January 2017.2018. In accordance with our policy, target annual bonus opportunities for all of our other NEOs in 20172018 were determined based on their positions as Executive Vice Presidents and, in the case of Mr. Covino, based on his position as Vice President and Chief Accounting Officer.Presidents.
| | | | |
| 42 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
The target annual bonus opportunity as a percentage ofyear-end annual base salary for each of our NEOs in 2018 compared to 2017 was as follows:
| | | | |
Name
| | 2017 Target %
| |
M. Vounatsos
| | | 125% | |
J. Capello(1)
| | | 70% | |
M. Ehlers
| | | 70% | |
S. Alexander
| | | 70% | |
P. McKenzie
| | | 70% | |
G. Covino
| | | 35% | |
G. Scangos(2)
| | | 140% | |
P. Clancy(3)
| | | 70% | |
K. DiPietro(3)
| | | 70% | |
(1) | Amount represents the target annual bonus opportunity for Mr. Capello. Based on his hire date, Mr. Capello was ineligible for payout under our 2017 annual bonus plan. |
(2) | Dr. Scangos ceased to be our Chief Executive Officer in January 2017, and received a prorated annual bonus payment pursuant to the severance provision of his employment agreement, as described in “2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Dr. Scangos Arrangements” above, based on actual Company performance and assuming 100% individual performance. |
(3) | Messrs. Clancy and DiPietro each ceased to be employed by the Company during 2017 and were ineligible for payouts under our 2017 annual bonus plan. |
| | | | | | | | |
| Name | | 2018 Target | | | 2017 Target | |
M. Vounatsos | | | 140% | | | | 125% | |
J. Capello | | | 70% | | | | 70% | |
M. Ehlers | | | 70% | | | | 70% | |
S. Alexander | | | 70% | | | | 70% | |
P. McKenzie | | | 70% | | | | 70% | |
20172018 Annual Bonus Plan Design
Awards for our NEOs under our 20172018 annual bonus plan were based on the achievement of Company performance goals and individual performance goals.
At the beginning of 2017,2018, our CompensationC&MD Committee set multiple Company performance goals for our 20172018 annual bonus plan and provided for a payout multiplier, which we refer to as the Company Multiplier, ranging from 0% to 150%, for each Company goal based on the determination of the level of achievement of each goal and application of the weighting previously assigned to each goal, which determined the Company Multiplier applied to the bonus calculation.
The Company Multiplier ranged from 0% to 150% as follows:
| | | | | | | | |
| | | | |
Performance Multipliers | | Below Threshold | | Threshold | | Target | | Max |
Company | | 0% | | 50% | | 100% | | 150% |
In addition, our 20172018 annual bonus plan payouts were also based on an assessment of each NEO’s individual performance, as compared totaking into account his or her achievement of individual performance goals. Our executive officers’ individual performance goals were discussed with and subject to our Compensation Committee’s approval. Our CEO’s individual goals were also approved by our Compensation Committee with input from the Chairman and the other independent directors. Evaluating individual performance allows our CompensationC&MD Committee the discretion to increase or decrease each NEO’s bonus amount based on the NEO’s individual performance by applying an individual performance multiplier, ranging from 0% to 150%, which we refer to as the Individual Multiplier.
| | | | |
41 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
We determined the individual annual bonus payments for 20172018 using the following calculation:
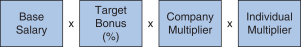
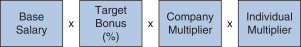
Our 20172018 annual bonus plan provided that if the Company Multiplier was less than 50%, there would be no payout, regardless of individual performance.performance, further strengthening ourpay-for-performance philosophy. Further, because the
Individual Multiplier and the Company Multiplier each have a maximum of 150%, the combined multiplier result for each NEO could not exceed 225%.
20172018 Company Performance Goals and Results
Company performance goals were established at the start of 20172018 with assigned weightings that reflected the Company’s focus on attaining both financial and strategic goals (pipeline performance, MS leadership, continued SMA launch excellence and enhancing our strategic alliances).
The goals and weightings we selected reflect the importance of linking reward opportunities to both near-term results and our progress in achieving longer-term goals.
The strategic goals we selected in 20172018 were designed to measure the achievement of our annual strategic priorities relating to our commercial opportunities and pipeline progress. Our financial performance goals were based on the Company’s annual operating plan and long-range plan approved by our Board of Directors and with reference to analyst consensus for Biogen revenues andNon-GAAP
diluted earnings per share (EPS)EPS based on the most current analyst reports at the time we set our targets.
The following table presents our financial targets relative to analysts’ consensus for 2017:2018:
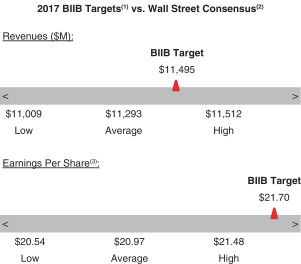
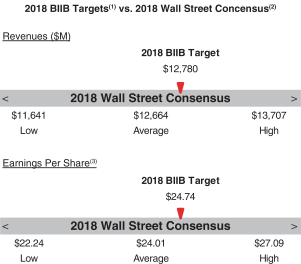
(1) See “2017Please see “2018 Annual Bonus Plan Company Performance Targets and Results Table” below for more details.
(2) Wall Street figures reflect estimates made in December 2016January 2018 for the Biogen fiscal year ending December 31, 2017.2018.
(3) ReflectsNon-GAAP Diluteddiluted EPS.
| | | | |
4243 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
20172018 Annual Bonus Plan Company Performance Targets and Results Table
Set forth below is a summary of the Company performance goals and weightings that our CompensationC&MD Committee established for our 20172018 annual bonus plan and the degree to which we attained these Company performance goals. As described below, the Company Multiplier for the 20172018 Annual Bonus Plan was 130%.131%, reflecting the strong performance relative to ourpre-established goals.
| | | | | | | | | | | | | | | | | | | Performance Range | | | | | | |
| | | | | Performance Range | | | | | | | | |
Company Goals | | Weight | | Threshold | | | Target | | | Max | | | Results | | | Company
Multiplier | | | Weight | | Threshold | | | Target | | | Max | | | Results | | Company Multiplier | |
FINANCIAL PERFORMANCE | | | | | | | | | | | | | | | | | | | | | | | | |
Revenues | | | | 20 | % | | $ | 12,310M | | | $ | 12,780M | | | $ | 13,250M | | | $ | 13,363M | (1) | | 150.0 | % |
Non-GAAP diluted EPS | | | 20 | % | | $ | 19.64 | | | $ | 21.70 | | | $ | 23.76 | | | $ | 23.19 | (1) | | | 123.0 | % | | | 20 | % | | $ | 23.47 | | | $ | 24.74 | | | $ | 26.01 | | | $ | 26.89 | (1) | | 150.0 | % |
Revenues | | | 20 | % | | $ | 10,687M | | | $ | 11,495M | | | $ | 12,303M | | | $ | 12,201M | (1) | | | 136.1 | % | |
MARKET PERFORMANCE | | | | | | | | | | | | | | | | | | | | |
Achieve U.S. SMA Market Share and Obtain SMA Approvals in E.U. and Certain Other Markets | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | | 131.3 | % | |
Achieve Global MS Market Share | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Below
Goal(2) |
| | | 96.5 | % | | | 15 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Below
Goal(2) |
| | 91.8 | % |
MS Leader in Customer Trust and Value Survey | | | 5 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Goal
Met |
| | | 100.0 | % | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | 125.0 | % |
Establish Three Outcome-based Innovative contracts | | | 5 | % | | | 1 | | | | 3 | | | | 5 | | | | 5 | | | | 150.0 | % | |
Achieve Global SMA Market Share | | | | 10 | % | |
| Specific market goals
are not disclosed for competitive reasons |
| |
| Above
Goal(2) |
| | 134.9 | % |
PIPELINE DEVELOPMENT | | | | | | | | | | | | | | | | | | | | |
Build and Advance Total Pipeline | | | 10 | % | |
| Specific pipeline goals are not
disclosed for competitive reasons |
| |
| Above
Goal(3) |
| | | 143.0 | % | | | 10 | % | |
| Specific pipeline goals
are not disclosed for competitive reasons |
| |
| Above
Goal(3) |
| | 110.0 | % |
Achieve Aducanumab Phase 3 Enrollment | | | 10 | % | |
| Specific enrollment goals are
not disclosed for competitive reasons |
| |
| Above
Goal(4) |
| | | 132.0 | % | | | 5 | % | |
| Specific enrollment goals
are not disclosed for competitive reasons |
| |
| Above
Goal(4) |
| | 105.0 | % |
COLLABORATION | | | | | | | | | | | | | | | | | | | | |
Improve Key Strategic Alliances | | | 10 | % | |
| Specific strategic
alliance goals are not
disclosed for competitive reasons |
| |
| Above
Goal(5) |
| | | 150.0 | % | |
Improve and Expand Key Strategic Alliances | | | | 10 | % | |
| Specific strategic
alliance goals are not
disclosed for competitive reasons |
| |
| Above
Goal(5) |
| | | 150.0 | % |
Company Multiplier | Company Multiplier | | | | 130.0 | %* | Company Multiplier | | | | 131.0 | %* |
| * | Numbers may not recalculate due to rounding. |
Notes to 20172018 Annual Bonus Plan Company Performance Targets and Results Table
| (1) | These financial measures were based on our publicly reported revenues of $12,274$13,453 million and our publicly announcedNon-GAAP diluted EPS of $21.81,$26.20, as adjusted as follows: for purposes of our 20172018 annual bonus plan, revenues andNon-GAAP diluted EPS were adjusted to neutralize the effects of foreign exchange rate fluctuations andfluctuations.Non-GAAP diluted EPS was further adjusted to add back $1.08$1.21 to reflect the impact of additional research and development expense recognized in 20172018 resulting from increased business development activitythe 2018 Ionis Agreement and $0.29$0.07 to neutralize the unfavorable impact of the worldwide withdrawal of ZINBRYTA, Article 20 Procedure,partially offset by the subtraction of $0.59 related to higher than originally contemplated stock repurchases in 2018, as these charges were not originally contemplated at the time the Company performance goals were determined. |
| (2) | Achievement of market goals for SMAMS was below goal and achievement of MS leader and market goals for SMA were above and below goals, respectively.goals. Specific details are not disclosed for competitive reasons. |
| (3) | The Company continued to expand andre-shape its pipeline ofpre-clinical and clinical stage programs through the advancement of internal programs, external business development activities and exceeding expectations with respect to the level of confidence in and momentum of its clinical stage portfolio. Specific details are not disclosed for competitive reasons. |
| (4) | Aducanumab Phase 3 clinical trial patient enrollment was above goal. Specific details are not disclosed for competitive reasons. |
| (5) | Key strategic alliance and acquisition activities were above goal. Specific details are not disclosed for competitive reasons. |
| | | | |
| 44 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
20172018 Individual Performance Goals and Results
The Individual Multiplier reflects each named executive officer’s overall individual performance rating as part of our performance assessment process. Unlike our formulaic calculation of corporate performance versusagainst Company per-
formanceperformance goals in determining the Company Multiplier, each named executive officer’s Individual Multiplier is based on a subjective evaluation of his or her overall performance and consideration of the achievement of individual goals established at the beginning of the year. Goals may be both quantitative and qualitative. For 2017,2018 Mr. Vounatsos recommended to our CompensationC&MD Committee an
| | | | |
43 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
Individual Multiplier for each current named executive officer other than himself based on his assessment of their individual contributions for the full year. Our CompensationC&MD Committee considered all of the information presented, discussed our CEO’s recommendations with him and its independent compensation consultant and applied its judgment to determine the Individual Multiplier for each current named executive officer. Our Board of Directors determined Mr. Vounatsos’ Individual Multiplier based on its assessment of his performance.
In its evaluation, our CompensationC&MD Committee assigned Individual Multipliers to our current named executive officers of between 130%115% and 145%140% based on the following accomplishments during 2017:2018:
Michel Vounatsos
Contributed to the achievement of record revenues of $12.3 billion$13,453 million and $26.20Non-GAAP diluted EPS for the year ended December 31, 2017.
Identified2018, versus targets of $12,780 million and took steps to create a leaner and simpler operating model to streamline our operations and reallocate resources towards prioritized research and development and commercial value creation opportunities, including the approval of a corporate restructuring program in October 2017.
Clarified and focused corporate strategy.
Made progress in defining and beginning to build a culture of excellence that values a tighter focus on priorities, faster decision making, enhanced accountability and improved teamwork.
Recruited outstanding members of our senior management team, including Dr. Gregory and Messrs. Capello and Guindo.$24.74, respectively.
Excelled in leading the Company in setting and achieving its financial goals and business development goals.
Added substantial value to our business development activities and the diversification of our pipeline.
Contributed significantly to the demonstrated resilience in our MS business, the continued successful launch of SPINRAZA worldwide.worldwide and the significant progress made in our biosimilars business.
Drove our ongoing improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values.
Jeffrey D. Capello
Contributed to the achievement of record revenues of $13,453 million and $26.20Non-GAAP diluted EPS for the year ended December 31, 2018, versus targets of $12,780 million and $24.74, respectively.
Significantly improved our Finance organization structure and key processes, including improved financial forecasting and planning and tax and treasury planning.
Added substantial value to our business development activities and the diversification of our pipeline.
Contributed to the return of approximately $4.4 billion to stockholders in 2018 through share repurchases under our 2018 Share Repurchase Program and 2016 Share Repurchase Program.
Contributed to excellent interactions with investors leading to transparent and trusted dialogue.
Contributed to improvements in our core processes to improve operating efficiencies, capital allocation and asset optimization while adhering to our core values.
Supported our Board of Directors, the CEO and executive team.
Michael Ehlers
Exceeded portfolio value and clinical development goals.
Significantly progressed and developed our pipeline.
Significantly improved our Research and Development organization structure, key processes and productivity.
Exceeded portfolio value and clinical development goals.
Added new capabilities and talent to our Research and Development organization.
Excelled in leadership of our Research and Development organization.
Added substantial value to our business development activities.
Contributed to excellent interactions with investors leading to transparent and trusted dialogue.
Susan H. Alexander
Supported our Board of Directors, the CEO and executive team transition and SEC disclosure requirements.
Led our initiative to create a leaner and simpler operating model to streamline our operations and reallocate resources towards prioritized research and development and commercial value creation opportunities, including the approval of a corporate restructuring program in October 2017.
Strengthened the intellectual property rights of our key assets, including our settlement and license agreement with Forward Pharma, pursuant to which we obtained U.S. and rest of world licenses to Forward Pharma’s intellectual property, including Forward Pharma’s intellectual property related to TECFIDERA.
Excelled in leadership of our Legal and Compliance teams while at the same time taking on leadership of our Corporate Services organization.teams.
Contributed significantly and excellently on strategy and the resolution of general business issues affecting the Company.Company, including our expansion into Asia Pacific and Latin America.
Supported the effective transition of the corporate services functions, including IT, to Mr. Capello.
Paul F. McKenzie
Excelled in management of our large and complex manufacturing organization.
Maintained excellence in manufacturing plant quality.
Contributed significantly to the successful launch of SPINRAZA worldwide.
Contributed significantly and excellently on strategy and general business issues affecting the Company.
Enhanced discipline and rigor with respect to decision making in our Pharmaceutical Operations & Technology organization.
Exhibited outstanding leadership, fostering a culture of continuous improvement and cost-consciousness.
Gregory F. Covino
Provided excellent leadership and support across our Finance organization following the departure of Mr. Clancy as our CFO in July 2017, including acting as our Interim Principal Financial Officer.
Supported the effective transition of Mr. Capello as our CFO.
Helped lead the Company in achieving its financial goals and performance.
Contributed significantly and excellently on strategy and general business issues affecting the Company.
Added substantial value to our business development activities.
| | | | |
44 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
In addition, our Compensation Committee reviews on a qualitative basis each named executive officer’s other contributions that are not covered by the individual performance
goals, leadership competencies and relative performance among our named executive officers.
2017 Annual Bonus Plan Awards
Our Compensation Committee determined that the final bonus awards under our 2017 annual bonus plan were as follows:
| | | | | | | | | | | | | | | | | | | | |
| Name | | Year-end Salary (A) x | | | Target Bonus% (B) x | | | Company
Multiplier (C) x | | | Individual Multiplier (D) = | | | Bonus Award (E) | |
M. Vounatsos | | $ | 1,100,000 | | | | 125 | % | | | 130 | % | | | 140 | % | | $ | 2,502,500 | |
J. Capello(1) | | $ | 750,000 | | | | 70 | % | | | n/a | | | | n/a | | | | n/a | |
M. Ehlers | | $ | 794,375 | | | | 70 | % | | | 130 | % | | | 140 | % | | $ | 1,012,034 | |
S. Alexander | | $ | 723,842 | | | | 70 | % | | | 130 | % | | | 130 | % | | $ | 856,305 | |
P. McKenzie | | $ | 603,750 | | | | 70 | % | | | 130 | % | | | 145 | % | | $ | 796,648 | |
G. Covino | | $ | 386,574 | | | | 35 | % | | | 130 | % | | | 140 | % | | $ | 246,248 | |
G. Scangos(2) | | $ | 1,500,000 | | | | 140 | % | | | n/a | | | | n/a | | | | n/a | |
P. Clancy(2) | | $ | 890,586 | | | | 70 | % | | | n/a | | | | n/a | | | | n/a | |
K. DiPietro(2) | | $ | 678,794 | | | | 70 | % | | | n/a | | | | n/a | | | | n/a | |
Notes to the 2017 Annual Bonus Plan Awards Table
(1) | Based on his hire date, Mr. Capello was ineligible for a payout under our 2017 annual bonus plan. |
(2) | Dr. Scangos received a prorated annual bonus payment pursuant to the severance provision of his employment agreement, as described in “2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Dr. Scangos’ Arrangements” above, based on actual Company performance and assuming an Individual Multiplier of 100%. Messrs. Clancy and DiPietro ceased to be employed by the Company during 2017 and were ineligible for payout under our 2017 annual bonus plan. |
| | | | |
| 45 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Excelled in leadership of our Pharmaceutical Operations and Technology organization.
Contributed significantly on strategy and the resolution of general business issues affecting the Company.
Contributed to the significant progress in our biosimilars business.
Exhibited outstanding leadership, fostering a culture of continuous improvement and cost-consciousness.
In addition, our C&MD Committee reviews on a qualitative basis each named executive officer’s other contributions to the Company and our business, leadership competencies and relative performance among our named executive officers.
2018 Annual Bonus Plan Awards
Our C&MD Committee determined that the final bonus awards under our 2018 annual bonus plan were as follows:
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
| Name | | Year-end Salary (A) x | | | Target Bonus% (B) x | | | Company Multiplier (C) x | | | Individual Multiplier (D) = | | | Bonus Award (E) | |
| | | | | |
| | | | | |
M. Vounatsos | | $ | 1,300,000 | | | | 140 | % | | | 131 | % | | | 140 | % | | $ | 3,337,880 | |
| | | | | |
| | | | | |
J. Capello | | $ | 750,000 | | | | 70 | % | | | 131 | % | | | 115 | % | | $ | 790,913 | |
| | | | | |
| | | | | |
M. Ehlers | | $ | 834,094 | | | | 70 | % | | | 131 | % | | | 120 | % | | $ | 917,837 | |
| | | | | |
| | | | | |
S. Alexander | | $ | 749,177 | | | | 70 | % | | | 131 | % | | | 125 | % | | $ | 858,744 | |
| | | | | |
| | | | | |
P. McKenzie | | $ | 633,938 | | | | 70 | % | | | 131 | % | | | 135 | % | | $ | 784,784 | |
| | | | | |
Long-Term Incentives (LTI)
| | | | | | |
| | |
| Terms | | Performance Stock Units (PSUs) | | Market Share Units (MSUs) |
| | |
Proportion of Annual Target Value | | 50% | | 50% |
| | |
| Settlement | | 60% stock settled | | 40% cash settled | | 100% stock settled |
| | | |
| Performance Period(s) | | 3 years (2018-2020) | | 1 year (each of 2018, 2019, 2020) | | 1 year, 2 years, 3 years (from grant date) |
| | | |
Metrics and Weighting | | AdjustedNon-GAAP diluted EPS: 30% Pipeline Milestone Performance: 30% | | Adjusted Free Cash Flow: 28% Revenues: 12% | | Stock Price: 100% |
| | | |
Threshold / Maximum Payout (% of Target Award) | | 50% / 200% | | 50% / 200% | | 50% / 200% |
| | | |
| Vesting | | 3-year Cliff Vesting | | 3-year Cliff Vesting | | Annual Ratable Vesting over 3 years (1/3 per year) |
| | | |
All annual LTI awards granted to our executives are performance-based and are designed to reward long-term Company performance.
Our executive annual LTI program for 20172018 consisted primarily of CSPUsPSUs and MSUs, with the annual LTI total target grant value of awards being split evenly between PSUs and MSUs. The CSPUsPSUs we awarded to executive officers are performance-based RSUs that may beare settled, as applicable, in cash or, in the discretion of our Compensation Committee,and shares of our common stock. The MSUs we awarded to executive officers are performance-based RSUs that are settled in shares of our common stock. The performance conditions applicable to these CSPUsPSUs and MSUs are described in further detail below. As used in this Proxy Statement, references to RSUs include CSPUs and MSUs. The
Our annual LTI awards are equally weighted between CSPUs and MSUs, based on grant date values.
We also generally award time-based RSUs in lieu of CSPUs at the time an executive is hired if employment commences after June 30th, as the performance period for CSPUs would be substantially in progress as of such time, and from time to time we grant time-based RSUs to recognize extraordinary contributions to the Company or in connection with new hires, as we did for Dr. McKenzie in 2017, or to recognize extraordinary contributions to the Company.
Our LTI planning range is reviewed each year. Our LTItarget grant values are differentiated based on an executive’s individual performance, potential future contributions and market competitiveness, as well as other factors. In determining the annual LTI planning range,target grant value, our CompensationC&MD Committee reviews our LTI planning ranges against target LTI awards of our peer group and also reviews the overall total compensation of our executive officers against our peer group due to ourgroup. In general, we have a heavier weighting in executive compensation mix towards LTI awards. No changes to our LTI planning range were made in 2017 as we believe that the current range positions us competitively against our peer group and allows for individual LTI award differentiation. On average, annual LTI target grant values for our NEOs (excluding Mr. Capello who joined in 2017 after the annual awards were granted and Mr. Covino based on his position) position their overalltotal compensation at or around the median values of our peer group in cases where there are comparable positions at the peer companies.
| | | | |
| 46 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
We have an established annual LTI grant practice where LTI grants are made following the completion of our internal performance reviews of our executive officers as well as our external market review of equity practices of our peer group, including the data from the Willis Towers Watson survey described above. Since 2004 we have made our annual LTI
grants in February of each year following our annual earnings release. Other
We generally grant time-based RSUs in lieu of PSUs at the time an executive is hired if employment commences after June 30th. These grants such as those made in connection with a new hire, are generally granted on the first trading day of the month following the date of hire. From time to time, we also grant time-based RSUs to recognize extraordinary contributions to the Company or for transition or retention purposes.
In 2017,2018 the annual LTI target grant date values for our NEOs were as follows:
| | | | |
| | |
| Name | | Annual LTI Grant
Target DateGrant Value
| | |
| | |
| | |
M. Vounatsos | | $10,000,00011,500,000 | | |
| | |
| | |
J. Capello(1) | | n/a | | |
| | |
| | |
M. Ehlers(2) | | $ 3,750,000 | | |
| | |
| | |
S. Alexander(2) | | $ 3,200,000 | | |
S. Alexander
| | $ 3,200,000 |
| | |
P. McKenzie(2) | | $ 2,750,000 | | |
G. Covino
| | $ 300,000 | | |
G. Scangos(3)
| | n/a | | |
P. Clancy(3)
| | $ 3,000,000 | | |
K. DiPietro(3)
| | $ 2,500,000 | | |
Notes to the 20172018 Annual LTI Awards Table
| (1) | Mr. Capello joined Biogen after theIn lieu of a 2018 annual LTI awards were granted.award, Mr. Capello received a new hire grant in January 2018, which consisted of PSUs and MSUs with an aggregate grant date target value of $3.0 million. BecauseThe initial determination of this grant, he was not eligible to receive an annual LTI award in 2018. With respect tothese awards took into account the PSUs, 60% (based on the grant date target value) will be settled in shares of our common stock and performance will be based upon achievement of cumulative three-year financial and pipeline metrics. The remaining 40% of the PSUs will be settled in cash and performance will be based upon the achievement of three annual financial metrics to be determined at the beginning of each relevant year. Please see “2018 LTI Program” below for additional information.Company’s peer group data. |
| (2) | In addition to the annual LTI award, Dr. Ehlers, Ms. Alexander and Dr. McKenzie each received aone-time transition award of RSUs. Please see “2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangements with Dr. McKenzie” above for a discussion of this additional LTI award. |
(3) | Dr. Scangos and Messrs. Clancy and DiPietro ceased to be employed by the Company during 2017. Based on his departure date, Dr. Scangos was not eligible for a 2017 grant. Mr. Clancy’s CSPU and MSU grants were subject to either accelerated or continued vesting,RSUs, as applicable, pursuant to the retirement provision under our 2008 Omnibus Equity Plan. Mr. DiPietro forfeited the majority of his 2017 grantdescribed in connection with the termination of his employment. Please see “2017 and 2018 Hiring- and Transition-Related Compensation Decisions” above for additional information.further detail below. |
The actual value that will be realized from CSPUPSU awards depends on the degree of achievement of performance goals, (revenues and adjusted free cash flow) applicable to each grant and the30-day average closing stock price on eachwith 60% of the dates such awards vest.PSUs (based on the grant date target value) settled in shares of our common stock based upon achievement of cumulative three-year financial and pipeline metrics and the remaining 40% of the PSUs settled in cash based upon the achievement of two annual financial metrics that are determined at the beginning of each relevant year. The actual value that will be realized from MSU awards depends on our30-day average common stock price growth between the grant date and each of the dates such awards vest. Our common stock price is influenced by the Company’s performance as well as external market factors.
2018 PSUs
PSUs comprised 50% of our executives’ target LTI for 2018. PSUs are performance-based RSUs that have three-year cliff vesting in furtherance of the Company’s long-termpay-for-performance philosophy and to encourage employee retention. PSUs align executive compensation to Company goals through performance against a combination of financial and pipeline milestone performance metrics. The actual value (if any) of PSUs will not be realized by the NEOs until the three-year period ends and then only if the applicable performance goals are achieved.
For our 2018 PSU awards, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock based on achievement of financial and pipeline performance goals over a three-year performance period (the 2018 Stock-Settled PSUs). The remaining 40% of the PSUs will be settled in cash based on the achievement of three sets ofone-year financial goals (the 2018 Cash-Settled PSUs) and continued employment through the vesting date. Our 2018 PSU awards are scheduled to vest in February 2021.
For our 2018 PSU awards, the number of PSUs earned at the end of the three-year performance period will be determined as follows:
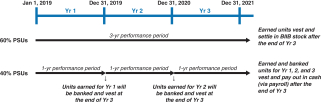
In designing our 2018 PSU LTI program, our C&MD Committee acknowledged the need to balance driving long-term performance and investing for the future with achieving key milestones along the way. Cash payments are primarily aligned with and reward more recent performance, while equity awards encourage our executives to continue to deliver results over a longer period of time and also serve as a retention tool. Accordingly, our C&MD Committee determined that moving compensation for our executive officers further away from cash and towards equity awards with longer-term goals would further align their interests with those of Biogen’s stockholders in creating long-term stockholder value.
| | | | |
4647 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
2018 PSU Awards Table
Set forth below is a summary of the performance metrics and weightings that our C&MD Committee established for our 2018 PSU awards and the degree to which we achieved the performance goals for the 2018 tranche of the 2018 Cash-Settled PSUs. Based on the results outlined in the table below, the multiplier for the 2018 tranche of the 2018 Cash-Settled PSUs was 192%.
| | | | | | | | | | | | | | | | |
| | | | | | |
Percentage of
PSU Award | | Percentage of PSU Target
Value / Total
LTI Target
Value | | Performance Metrics | | Performance
Metrics
Weight | | | Performance
Period | | | Target Performance | | Actual Performance |
Stock- Settled: 60% | | 60% / 30% | | Adjusted Non-GAAP diluted EPS Pipeline Milestone Performance | |
| 30%
30% |
| |
| 2018-2020
2018-2020 |
| | Specific goals are not disclosed for competitive reasons |
Cash- Settled: 40% | | 40% / 20% | | Adjusted Free Cash Flows Revenues | | | 28% 12% | | |
| 2018
2019 2020 2018 2019 2020 |
| | $ 2.9B Target set at beginning of 2019 Target set at beginning of 2020 $ 12.8B Target set at beginning of 2019 Target set at beginning of 2020 | | $ 4.0B(1)
TBDTBD $ 13.4B(2)
TBD TBD |
Notes to the 2018 PSU Awards Table
| (1) | This financial measure was based on ourNon-GAAP free cash flows, as adjusted to add back $256 million to reflect the cash impact of additional research and development expense recognized in 2018 resulting from the 2018 Ionis Agreement, $16 million to neutralize the unfavorable cash impact of the worldwide withdrawal of ZINBRYTA and $33 million related to higher than originally contemplated stock repurchases in 2018, partially offset by the subtraction of $235 million to reflect tax payments made in connection with tax reform, as these charges were not originally contemplated at the time these performance goals were determined. |
| (2) | This financial measure was based on our publicly reported revenues of $13.5 billion, as adjusted to neutralize the effects of foreign exchange rate fluctuations. |
average common stock price growth between the grant date andThe 2018 Stock-Settled PSUs metrics were approved by our C&MD Committee with equal weighting assigned to each of the dates such awards vest. Our common stock price is influenced by the Company’s performance as well as external market factors.
2017 CSPUs
CSPUs are performance-based RSUs that are subject to aone-year performance period and three-year service-based vesting. Our 2017 CSPU awards are earned and eligible to vest based uponmetric. The two metrics selected were the achievement of an equal weightinga cumulative three-year adjustedNon-GAAP diluted EPS and pipeline milestone performance, in each case, for the three-year period of revenues and adjusted free cash flow results when compared to2018 through 2020.
Adjustedpre-establishedNon-GAAP performance goals setdiluted EPS measured at the
startend of
thethree-year performance period
by our Compensation Committee.Revenues was selected as in past years, and is the same financial measure utilized in the determination of the 2017 annual cash bonuses. We selected revenues as a performance measure to reinforce the importance of achieving long-term financial and exceedingoperational performance. Our C&MD Committee believes that adjustedNon-GAAP diluted EPS is a transparent, operations-based measure.
Pipeline milestone performance over the three-year period of 2018 through 2020 was selected to drive our revenue goallong-term strategic direction and to provide further incentive to achieve such goal.stockholder value creation through our pipeline progress.
We also selected anThe 2018 Cash-Settled PSUs financial metrics are adjusted free cash flowflows and revenues. At the beginning of each year during the performance measure, similar to past years,period for our 2018 PSU awards,
our C&MD Committee will approve the targets for each of these financial metrics for such year. Our C&MD Committee decided that because of the nature of our Compensationbusiness, in which operating metrics can potentially be impacted positively or negatively by events outside of the control of executives, the design of the PSU program would be based, in part, on the use of threeone-year financial goals.
Our C&MD Committee views free cash flow as a critical measure to align the interests of management with those of our stockholders as it reflects the net cash flows available to the Company to pursue opportunities that enhance stockholder value. We believe that long-term cash flow generation of our Company best reflects the intrinsic value of our enterprise. As such, a cash flow performance goal encourages management to optimize capital expenditures, invest prudently in high return projects and optimize working capital.
We selected revenues as a performance measure to reinforce the importance of achieving and exceeding our revenue goal and to provide further incentive to achieve such goal.
| | | | |
| 48 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
In order to further motivate our executives to drive the organization toward the achievement of these goals, we provide for a maximum payout of 200%. for our 2018 PSU awards. Participants may ultimately earn between 0% and 200% of the target number of CSPUsPSUs granted based on the degree of actual performance goal achievement. Earned CSPUs are subject to service-based vesting, withone-third of the earned CSPUs vesting on each of the first three anniversaries of the grant date,achievement, generally subject to continued service with the Company.
2017 CSPU Company Performance Targets and Results Table2018 MSUs
The following table shows thepre-established corporate performance goals and the actual results that determined the percentage of target CSPUs earned for 2017:
| | | | | | | | | | | | | | |
| Company Goals (1) | | Weight % | | Target Performance Range | | | | Payout | | |
| | | Threshold | | Target | | Max | | Results | | |
Revenue | | 50% | | $10,687M | | $11,495M | | $12,604M | | $12,201M | | 136.1% | | |
Adjusted Free Cash Flow | | 50% | | $3,671M | | $4,110M | | $4,714M | | $4,285M | | 109.6% | | |
CSPU Performance Multiplier | | | | 123.0%* | | |
* | Numbers may not recalculate due to rounding. |
Notes to 2017 CSPU Company Performance Targets and Results Table
(1) See “Notes to 2017 Annual Bonus Plan Performance Company Targets and Results Table” above for definitions and adjustments related to revenue goals and results.
(2) Final 2017 adjusted free cash flow was adjusted to remove (a) the net of tax impact of $366.0 million of operating charges in excess of $100.0 million recognized in relation to upfront and developmental milestones and other expenses associated with our agreements to exclusively license BIIB092 and BIIB098 and entering into a new collaboration agreement with Ionis to identify new ASO drug candidates for the treatment of SMA and (b) the net of tax impact of $61.0 million related to the Article 20 Procedure of ZINBRYTA and resulting impairment of ZINBRYTA related assets.
The 2017 CSPUs are also subject to stock price performance, in that the value actually received in respect of CSPUs is dependent on the performanceMSUs comprised 50% of our common stock, and are subject to service-based vesting over three years from the grant date, in furtherance of the Company’s long-termpay-for-performance philosophy and to encourage employee retention.
Once vested, the CSPUs are generally converted into and settled in cash, based on the30-day average closing price of our common stock prior to and including the date on which they vest, except that, with respect to equity awards made to the executive officers, our Compensation Committee may, in its discretion, settle such awards in shares of our common stock or in cash.
| | | | |
47 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued) |
CSPU Illustration:
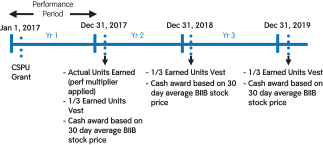
2017 MSUs
executives’ target LTI for 2018. MSUs are performance-based RSUs that are earned based on the growth of our common stock price performance from the date of grant to each of the three annual vesting dates. On each vesting date, the performance multiplier is derived based on the stock price growth measured from the grant date to such vesting date using the average closing stock price for the 30 calendar days prior tofollowing and including the grant date and 30 calendar days prior to and including such vesting date. The performance multiplierdate for MSUs awarded prior to 2014 continues to be calculated using a60-day average closing stock price and vesting occurs over 4 annual installments.granted in 2018.
Participants may ultimately earn between 0% and 200% of the target number of MSUs awarded based on actual stock performance. The maximum payout percentage of MSUs available to be awardedgranted in 20172018 is consistent with thethose granted in 2017 CSPUs (200%). Once the performance multiplier is determined, it is applied to the target number of MSUs granted to each executive and can increase or decrease the overall number of MSUs earned based on stock price performance. For grants made prior to 2014, the maximum payout continues to be 150%.
MSU Illustration:Illustration
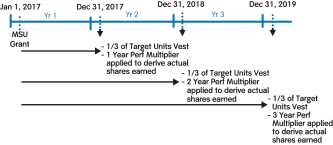
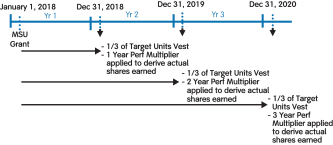
The three-year (or four year, forpre-2014 MSUs) service vesting period ties executive compensation directly to our common stock price performance, as both the unitsMSUs earned
and the value actually received in respect of MSUs isare dependent on the performance of our common stock.stock over the vesting period. On each vesting date, the earned MSUs are settled in shares of our common stock.
The following table shows the vesting date, performance period and performance multiplier applied for MSUs vesting in 20172018 and 2018:2019:
| | | | | | | | | | |
| | | |
| Grant Date | | VestVesting
Date | | Performance Period | | | Performance Multiplier | |
| | | |
2/2018 | | 2/2019 | | | 1 year | | | | 114% | |
| | | |
2/2017 | | 2/2019 | | | 2 years | | | | 124% | |
| | 2/2018 | | | 1 year | | | | 126% | |
| | | |
2/2016 | | 2/2019 | | | 3 years | | | | 134% | |
| | 2/2018 | | | 2 years | | | | 132% | |
| 2/2017 | | | 1 year | | | | 111% | |
|
2/2015
| 2/2018 | | | 3 years | | | | 88% | | | 2/2017 | | | 2 years | | | | 75% | |
2/2014
| | 2/2017 | | | 3 years | | | | 92% | |
2/2013
| | 2/2017 | | | 4 years | | | | 150% | |
2018 LTI ProgramOne-Time Transition Awards
Although we believe our executive compensation programs are effectively designed and have worked well to implement apay-for-performance culture aligned with the interests of our stockholders, our Compensation Committee decided to make certain enhancements to the design of our 2018 LTI program to reinforce focus on long-term performance. Specifically, under our 2018 LTI program, grants of PSUs have replaced CSPUs. The PSUs will have three-year cliff vesting. In addition, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock and performance will be based upon achievement of cumulative three-year financial and pipeline metrics. The remaining 40% of the PSUs will be settled in cash and performance will be based upon the achievement of three annual financial metrics to be determined at the beginning of each relevant year.
As part of our 2018 LTI program change and transition plan, our CompensationC&MD Committee also decided to grantone-time special transition awards in the form of time-based RSUs to certain eligible executive officers. These awards were granted in February 2018 to certain executive officers, excluding the CEO,Messrs. Vounatsos and willCapello, which vest over atwo-year period, with 33% vesting on the first anniversary of the grant date and 67% vesting on the second anniversary of the grant date. Our Compensation Committee decidedThese awards were intended to grant these awards in acknowledgement ofhelp mitigate the impact on executives’ compensation and cash flow disruption due to the program changes, including the change to the three-year cliff vesting schedule applied to the PSU awards discussed above compared to the three-yearannual installment vesting over three years that previously applied to CSPUs.
| | | | |
48 | |  | |  |
In 2018 theone-time transition awards of RSUs for our NEOs were as follows:
| | |
5 | |
| Name | | Grant Executive Compensation Matters (continued)Date Value
|
| |
| |
M. Vounatsos | | n/a |
| |
| |
J. Capello | | n/a |
| |
| |
M. Ehlers | | $ 1,500,000 |
| |
| |
S. Alexander | | $ 1,280,000 |
| |
| |
P. McKenzie | | $ 1,200,000 |
| |
Retirement Plans
We maintain a Supplemental Savings Plan (SSP), which is anon-qualified deferred compensation plan covering our executive officers and other eligible employees in the U.S. We offer the SSP as part of the retirement savings component of our benefits program. We designed the SSP to be competitive with thenon-qualified deferred compensation plans offered by companies in our peer group.group at that time. Details of the SSP are discussed under the heading “2017“2018Non-Qualified Deferred Compensation” below.
| | | | |
| 49 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Other Benefits
In addition to eligibility for the benefit programs generally provided to all employees, such as our employee stock purchase plan, 401(k) plan and medical, dental, vision, life and disability insurance, we provide certain supplemental benefits to our executives. These benefits include:
Life Insurance
All of our U.S. executives, including our NEOs, receive Company-paid term life insurance equal to three times their annual base salary, up to a maximum benefit ofamount. In 2018 the maximum benefit amount for the CEO was $1.5 million.million and was $2.25 million for the other NEOs. Employees who are not executives receive Company-paid term life insurance equal to two times their annual base salary. The additional value of Company-provided life insurance for our executive officers reflects competitive practices and is consistent with our philosophy to provide appropriate levels of financial security for our employees based on their positions within the Company. The cost of Company-paid life insurance in excess of a $50,000 insurance level is taxable income to U.S. employees and is not grossed up by the Company.
Executive Physicals, Tax Preparation, Financial and Estate Planning
Our executive officers, other than our CEO, are eligible for reimbursement of expenses incurred for tax preparation and financial and estate planning services, as well as the purchase of tax preparation and financial planning software, subject to annual expense limits of $7,500 for executive vice presidents and $4,500 for vice presidents.Executive Vice Presidents. Such reimbursements are taxable income to our executives and are not grossed up.
All of our executive officers, including our CEO, are eligible for reimbursement for the cost of their executive physicals, subject to the annual expense limits noted above of $7,500
for our executive vice presidentsExecutive Vice Presidents and CEO and $4,500 for vice presidents.CEO. This benefit provides our executives with additional flexibility to proactively manage their health and wellness.
Relocation Expenses
Under our Executive Relocation Policy, we will, in certain circumstances, provide relocation benefits when executivesemployees first join us.
Post-Termination Compensation and Benefits
We provide severance benefits to all of our executive officers if they are terminated without cause or in certain other
circumstances. The terms of these arrangements and the amounts payable under them are described below for each NEO under the heading “Potential Payments Upon Termination or Change in Control.” We provide these benefits because we believe that severance protection is necessary to help our executives maintain their focus on the best interests of the Company when providing advice to the Company and when making strategic decisions about a potential corporate transaction or change in control, and further encourages effective leadership in the closing and integration of significant transactions affecting the Company.
Stock Ownership Guidelines
We maintain stock ownership guidelines for our executive officers to strengthen and reinforce the link our compensation programs create between our executives and our stockholders. A summary of our stock ownership guidelines is set forth below.
| | |
| |
| Level | | Number of Shares Equal in Value to: |
| |
CEO | | 6x base salary |
| |
| |
EVPExecutive Vice Presidents | | 3x base salary |
Chief Accounting Officer
| | 1x salary |
Executive officers have five years from their initial appointment to meet the requirement. In the event the requirement is not met within that time, 100% of vested shares received in respect of LTI awards are required to be held until the requirement is satisfied. Only stock owned outright or otherwise vested or earned performance-based shares is credited toward the stock ownership requirement. Shares underlying unvested RSUsor unearned performance-based shares are not included in the calculation. All of our executive officers currently meet the stock ownership requirement or are still within the five-year period to meet such requirement.
| | | | |
49 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued)
|
Recoupment of Compensation
We may recover compensation from our employees, including our executive officers, who engage in detrimental or competitive activity. Detrimental activity includes any action or failure to act that constitutes financial malfeasance that is materially injurious to the Company, violates our Code of Business Conduct (Values in Action), results in a restatement of our earnings or financial results or results in a violation or breach of law or contract. Competitive activity includes any action or failure to act that violatesnon-disclosure,non-competition and/ornon-solicitation agreements. Our 2008 Performance-Based Management Incentive Plan
| | | | |
| 50 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
allows for the forfeiture and/or repayment of cash-based awards and our 2008 Omnibus Equity Plan and our 2017 Omnibus Equity Plan each allow for the cancellation of LTI awards in these circumstances.circumstances as well as the forfeiture of stock or cash acquired upon vesting or sale of LTI awards. In addition, cashsign-on bonuses paid to our NEOs may be subject to repayment if the NEO voluntarily resigns from the Company or if his or her employment is terminated by the Company in certain circumstances.
Insider Trading, Hedging and Pledging Policy Prohibitions
We maintain a Global Insider Trading and Information Policy that prohibits our employees and directors from, among other things, engaging in hedging or derivative transactions with respect to the Company’s equity securities, purchasing Company stock on margin, pledging Company securities as collateral for a loan or engaging in short sales of the Company’s securities.
Tax-Deductibility of Compensation
Section 162(m) of the Internal Revenue Code (Section 162(m))generally limits the amount a company may deduct for compensation in excess of $1 million paid to certain “covered employees”. For taxable years ending December 31, 2017, and earlier, “covered employees” generally referredemployees,” subject to the chief executive officer and the next three highly compensated executive officers (excluding the chief financial officer). However, for these taxable years this limitation did not apply to compensation meeting the definition of qualifying performance-based compensation.
The exemption from Section 162(m)’s deduction limitation for qualifying performance-based compensation has been repealed by recent legislation, effective for taxable years
beginning after December 31, 2017, such that compensation paid to covered employees in excess of $1 million will not be deductible unless it qualifies forcertain transition relief applicable to certain arrangements in place as of November 2, 2017. In addition, for taxable years beginning2017, and not materially modified after December 31, 2017, “covered employee” generally has been expanded to include a company’s chief financial officer. In addition, each individual who is a covered employee for any taxable year beginning after December 31, 2016 will remain a covered employee for all future years.such date.
ManagementOur C&MD Committee regularly reviews the provisions of our plans and programs, monitors legal developments and works with our Compensation Committee and its independent compensation consultant to review and consider Section 162(m)reviews and considers,
among other things, the tax deductibility of compensation payments. Our CompensationC&MD Committee, however, believes that compensation programs that attract, retain and reward executive talent and achievement are necessary for our success and, therefore, are in the best interests of the Company and our stockholders and that, in establishing the cash and equity incentive compensation programs for the Company’s executive officers, the potential deductibility of the compensation payable under such programs should only be one of a number of relevant factors taken into consideration. Consequently, our CompensationC&MD Committee may pay or provide, and has paid or provided, compensation in excess of $1.0 million that is not exempt from the deduction limitations under Section 162(m).tax deductible in whole or in part.
Compensation Committee Report
The Compensation and Management Development Committee furnishes the following report:
The Compensation and Management Development Committee has reviewed and discussed the Compensation Discussion and Analysis with Biogen management. Based on this review and discussion, the Compensation and Management Development Committee recommended to ourthe Board of Directors that the Compensation Discussion and Analysis be included in this Proxy Statement.
Submitted by,
Robert W. Pangia (Chair)
Richard C. Mulligan
Eric K. Rowinsky
Lynn Schenk
| | | | |
5051 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Summary Compensation Table
The following table shows the compensation paid to or earned by our NEOs during the years ended December 31, 2017,2018, December 31, 2016,2017, and December 31, 2015,2016, for the year(s) in which they were a named executive officer.
| | Name and Principal Position (a) | | Year (b) | | Salary (c) | | Bonus(1) (d) | | Stock Awards(2) (e) | | Non-Equity Incentive Plan Compensation(3) (f) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings(4) (g) | | All Other Compensation(5) (h) | | Total (i) | | | Year (b) | | Salary (c) | | Bonus(1) (d) | | Stock Awards(2) (e) | | Non-Equity Incentive Plan Compensation(3) (f) | | Change in Pension Value and Nonqualified Deferred Compensation Earnings(4) (g) | | All Other Compensation(5) (h) | | Total (i) |
Michel Vounatsos(6) | | | 2017 | | | $ | 1,087,885 | | | | — | | | $ | 9,868,540 | | | $ | 2,502,500 | | | $ | 18,881 | | | $ | 186,567 | | | $ | 13,664,373 | | | | 2018 | | | $ | 1,276,923 | | | | — | | | $ | 11,064,897 | | | | $3,337,880 | | | | $ 80,663 | | | | $408,283 | | | $ | 16,168,646 | |
Chief Executive Officer | | 2016 | | | $ | 519,231 | | | $ | 1,500,000 | | | $ | 3,151,199 | | | $ | 447,799 | | | $ | 1,598 | | | $ | 181,568 | | | $ | 5,801,395 | | | | 2017 | | | $ | 1,087,885 | | | | — | | | $ | 9,868,540 | | | | $2,502,500 | | | | $ 18,881 | | | | $186,567 | | | $ | 13,664,373 | |
| | | | 2016 | | | $ | 519,231 | | | $ | 1,500,000 | | | $ | 3,151,199 | | | | $ 447,799 | | | | $ 1,598 | | | | $181,568 | | | $ | 5,801,395 | |
Jeffrey D. Capello(7) | | | 2017 | | | $ | 28,846 | | | $ | 520,000 | | | | — | | | | — | | | | — | | | $ | 132 | | | $ | 548,978 | | | | 2018 | | | $ | 750,000 | | | | — | | | $ | 2,889,224 | | | | $ 790,913 | | | | — | | | | $ 46,582 | | | $ | 4,476,719 | |
Executive Vice President and Chief Financial Officer | | | | | | | | | | | | | | | | | |
Executive Vice President | | | | 2017 | | | $ | 28,846 | | | $ | 520,000 | | | | — | | | | — | | | | — | | | | $ 132 | | | $ | 548,978 | |
| and Chief Financial Officer | | |
Michael D. Ehlers | | | 2017 | | | $ | 792,139 | | | | — | | | $ | 3,157,338 | | | $ | 1,012,034 | | | $ | 1,799 | | | $ | 101,671 | | | $ | 5,064,981 | | | | 2018 | | | $ | 829,511 | | | | — | | | $ | 5,107,535 | | | | $ 917,837 | | | | $ 5,258 | | | | $186,510 | | | $ | 7,046,651 | |
Executive Vice President, | | 2016 | | | $ | 491,827 | | | $ | 1,170,177 | | | $ | 3,410,650 | | | $ | 425,062 | | | $ | 155 | | | $ | 14,665 | | | $ | 5,512,536 | | | | 2017 | | | $ | 792,139 | | | | — | | | $ | 3,157,338 | | | | $1,012,034 | | | | $ 1,799 | | | | $101,671 | | | $ | 5,064,981 | |
Research & Development | | | | | | | | | | | | | | | | | | | 2016 | | | $ | 491,827 | | | $ | 1,170,177 | | | $ | 3,410,650 | | | | $ 425,062 | | | | $ 155 | | | | $ 14,665 | | | $ | 5,512,536 | |
Susan H. Alexander | | | 2017 | | | $ | 720,630 | | | | — | | | $ | 3,157,338 | | | $ | 856,305 | | | $ | 148,961 | | | $ | 178,008 | | | $ | 5,061,242 | | | | 2018 | | | $ | 746,254 | | | | — | | | $ | 4,359,590 | | | | $ 858,744 | | | | $188,056 | | | | $201,140 | | | $ | 6,353,784 | |
Executive Vice President, | | 2016 | | | $ | 693,663 | | | | — | | | $ | 2,337,051 | | | $ | 589,514 | | | $ | 133,726 | | | $ | 171,114 | | | $ | 3,925,068 | | | | 2017 | | | $ | 720,630 | | | | — | | | $ | 3,157,338 | | | | $ 856,305 | | | | $148,961 | | | | $178,008 | | | $ | 5,061,242 | |
Chief Legal Officer | | 2015 | | | $ | 697,721 | | | | — | | | $ | 2,795,055 | | | $ | 266,069 | | | $ | 112,406 | | | $ | 296,052 | | | $ | 4,167,303 | | | | 2016 | | | $ | 693,663 | | | | — | | | $ | 2,337,051 | | | | $ 589,514 | | | | $133,726 | | | | $171,114 | | | $ | 3,925,068 | |
and Secretary | | | | | | | | | | | | | | | | | |
Paul F. McKenzie | | | 2017 | | | $ | 600,433 | | | | — | | | $ | 3,514,451 | | | $ | 796,648 | | | $ | 2,471 | | | $ | 101,254 | | | $ | 5,015,257 | | | | 2018 | | | $ | 630,455 | | | | — | | | $ | 4,083,884 | | | | $ 784,784 | | | | $ 12,367 | | | | $154,406 | | | $ | 5,665,896 | |
Executive Vice President, | | | | | | | | | | | | | | | | | | | 2017 | | | $ | 600,433 | | | | — | | | $ | 3,514,451 | | | | $ 796,648 | | | | $ 2,471 | | | | $101,254 | | | $ | 5,015,257 | |
Pharmaceutical Operations & Technology | | | | | | | | | | | | | | | | | |
Gregory F. Covino(8) | | | 2017 | | | $ | 385,275 | | | | — | | | $ | 297,750 | | | $ | 246,248 | | | $ | 22,787 | | | $ | 43,470 | | | $ | 995,530 | | |
Vice President, Finance and Chief Accounting Officer and Former Interim Principal Financial Officer | | | | | | | | | | | | | | | | | |
George A. Scangos(9) | | | 2017 | | | $ | 60,065 | | | | — | | | | — | | | | — | | | $ | 159,968 | | | $ | 7,255,796 | | | $ | 7,475,829 | | |
Former Chief Executive | | 2016 | | | $ | 1,500,000 | | | | — | | | $ | 13,007,653 | | | $ | 2,541,000 | | | $ | 221,642 | | | $ | 463,493 | | | $ | 17,733,788 | | |
Officer | | 2015 | | | $ | 1,538,462 | | | | — | | | $ | 13,015,232 | | | $ | 1,181,250 | | | $ | 184,724 | | | $ | 954,718 | | | $ | 16,874,386 | | |
Paul J. Clancy(10) | | | 2017 | | | $ | 458,945 | | | | — | | | $ | 2,961,929 | | | | — | | | $ | 61,983 | | | $ | 168,361 | | | $ | 3,651,218 | | |
Former Executive Vice | | 2016 | | | $ | 844,600 | | | | — | | | $ | 3,556,773 | | | $ | 728,818 | | | $ | 55,376 | | | $ | 199,635 | | | $ | 5,385,202 | | |
President, Finance and | | 2015 | | | $ | 747,498 | | | | — | | | $ | 2,543,374 | | | $ | 284,655 | | | $ | 45,960 | | | $ | 332,115 | | | $ | 3,953,602 | | |
Chief Financial Officer | | | | | | | | | | | | | | | | | |
Kenneth A. DiPietro(11) | | | 2017 | | | $ | 568,319 | | | | — | | | $ | 2,467,730 | | | | — | | | $ | 149 | | | $ | 2,187,069 | | | $ | 5,223,267 | | |
Former Executive Vice | | 2016 | | | $ | 652,581 | | | | — | | | $ | 2,539,625 | | | $ | 555,496 | | | $ | 18,011 | | | $ | 174,714 | | | $ | 3,940,427 | | |
President, Human | | 2015 | | | $ | 648,023 | | | | — | | | $ | 2,795,055 | | | $ | 247,117 | | | $ | 12,081 | | | $ | 287,621 | | | $ | 3,989,897 | | |
Resources | | | | | | | | | | | | | | | | | |
Notes to the Summary Compensation Table
| (1) | The amounts in column (d) reflectsign-on bonuses paid to Mr.Messrs. Vounatsos Mr.and Capello and Dr. Ehlers at the time of hire. All other cash bonuses, which were based on achievement of performance criteriagoals under our annual bonus plan, are disclosed in column (f). |
| (2) | The amounts in column (e) reflect the grant date fair value computed in accordance with ASC 718 for RSUs, MSUs, PSUs and CSPUs granted during 2018, 2017 2016 and 2015,2016, as applicable, excluding the effect of estimated forfeitures. The cash portion of PSUs are included in the year when goals are set and fair value is determinable. The 2018 amounts includeone-third of the 2018 Cash-Settled PSUs, which is the tranche of the award for which goals had been set in 2018 relating to the 2018 performance period. The 2018 amounts also includeone-time transition awards of RSUs granted to each of the NEOs, except Messrs. Vounatsos and Capello, as described under “2018One-Time Transition Awards” above. The 2017 amounts for Dr. McKenzie represent grants of MSUs, CSPUs and RSUs, as described in more detail“Executive Compensation Matters—Compensation Discussion and Analysis—2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangement with Dr. McKenzie” in the CD&A above, and theour 2018 proxy statement. The 2016 amounts for Dr. Ehlers represent grants of MSUs, CSPUs and RSUs as described in “Executive Compensation Matters—Compensation Discussion and Analysis—2016 and 2017 Hiring- and Transition-Related Compensation Decisions—Arrangements with Mr. Vounatsos and Dr. Ehlers” in our 2017 proxy statement. The amounts for all other NEOs for 2017 and, as applicable, for 2016 and 2015 represent grants of MSUs and CSPUs. The awards granted before February 1, 2017, were subsequently adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts reported in this column were not impacted by such anti-dilution adjustments. The grant date fair value for MSU awards are estimated as of the date of grant using a lattice model with a Monte Carlo simulation, based on the probable outcome of applicable performance conditions, on the date of grant. Assumptions used in this calculation are included on pageF-46 in footnote 16 of our 2017 Annual Report on Form10-K.conditions. The grant date fair value for PSU, CSPU and RSU awards was determined by multiplying the number of shares subject to the award (assuming target performance for PSUs and CSPUs) by the closing price of the Company’s common stock on the grant date. The assumptions used to calculate the grant date fair value of stock awards are included in footnote 16 of our 2018 Annual Report on Form10-K. The table below shows the target and maximum payouts possible for the 2018, 2017 and 2016 MSU, PSU and CSPU awards based on the value at the date of grant and the target and maximum payout levels. |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | |
| | | 2018 | | | 2017 | | | 2016 | |
| | | | | | |
| Executive Officer | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | |
Mr. Vounatsos | | | $11,064,897 | | | | $22,129,794 | | | | $9,868,540 | | | | $19,737,080 | | | | $3,151,199 | | | | $6,302,398 | |
Mr. Capello | | | $ 2,889,224 | | | | $ 5,778,448 | | | | — | | | | — | | | | — | | | | — | |
Dr. Ehlers | | | $ 3,608,292 | | | | $ 7,216,584 | | | | $3,157,338 | | | | $ 6,314,676 | | | | $2,540,438 | | | | $5,080,876 | |
Ms. Alexander | | | $ 3,078,822 | | | | $ 6,157,644 | | | | $3,157,338 | | | | $ 6,314,676 | | | | $2,337,051 | | | | $4,674,102 | |
Dr. McKenzie | | | $ 2,883,856 | | | | $ 5,767,712 | | | | $2,713,851 | | | | $ 5,427,702 | | | | — | | | | — | |
| | | | |
5152 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
| target and maximum payouts possible for the 2017, 2016 and 2015 MSU and CSPU awards based on the value at the date of grant and the target and maximum payout levels. |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2017 | | | 2016 | | | 2015 | |
| Executive Officer | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | | | Target Payout | | | Maximum Payout | |
Mr. Vounatsos | | $ | 9,868,540 | | | $ | 19,737,080 | | | $ | 3,151,199 | | | $ | 6,302,398 | | | | — | | | | — | |
Mr. Capello | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Dr. Ehlers | | $ | 3,157,338 | | | $ | 6,314,676 | | | $ | 2,540,438 | | | $ | 5,080,876 | | | | — | | | | — | |
Ms. Alexander | | $ | 3,157,338 | | | $ | 6,314,676 | | | $ | 2,337,051 | | | $ | 4,674,102 | | | $ | 2,795,055 | | | $ | 5,590,110 | |
Dr. McKenzie | | $ | 2,713,851 | | | $ | 5,427,702 | | | | — | | | | — | | | | — | | | | — | |
Mr. Covino | | $ | 297,750 | | | $ | 595,500 | | | | — | | | | — | | | | — | | | | — | |
Dr. Scangos | | | — | | | | — | | | $ | 13,007,653 | | | $ | 26,015,306 | | | $ | 13,015,232 | | | $ | 26,030,464 | |
Mr. Clancy | | $ | 2,961,929 | | | $ | 5,923,858 | | | $ | 3,556,773 | | | $ | 7,113,546 | | | $ | 2,543,374 | | | $ | 5,086,749 | |
Mr. DiPietro | | $ | 2,467,730 | | | $ | 4,935,460 | | | $ | 2,539,625 | | | $ | 5,079,250 | | | $ | 2,795,055 | | | $ | 5,590,110 | |
| (3) | The amounts in column (f) reflect actual bonuses paid under our annual bonus plan for the applicable year. |
| (4) | The amounts in column (g) reflect earnings in the SSP that are in excess of 120% of the applicable federal long-term rate. The federal long-term rates applied in this calculation are 3.26%3.06%, 3.26% and 3.14% for 2018, 2017 and 3.16% for 2017, 2016, and 2015, respectively. A description of theThe SSP is described under the heading “2017“2018Non-Qualified Deferred Compensation” below. |
| (5) | The amounts in column (h) for 20172018 reflect the following: |
| | | Executive Officer | | Company Matching Contribution to 401(k) Plan Account (12) | | | Company Contribution to SSP Account | | | Personal Health and Financial Planning(13) | | | Value of Company- Paid Life Insurance Premiums | | | Relocation(14) | | | Recognition
Award(15) | | | Severance | | | Company Matching Contribution to 401(k) Plan Account | | Company Contribution to SSP Account | | Personal Health and Financial Planning(8) | | Value of Company- Paid Life Insurance Premiums | | Relocation(9) |
Mr. Vounatsos | | $ | 17,931 | | | $ | 113,911 | | | | — | | | $ | 1,055 | | | $ | 53,670 | | | | — | | | | — | | | $ | 16,500 | | | $ | 387,134 | | | $ | 3,594 | | | $ | 1,055 | | | | — | |
Mr. Capello | | | — | | | | — | | | | — | | | $ | 132 | | | | — | | | | — | | | | — | | | $ | 16,500 | | | $ | 28,500 | | | | — | | | $ | 1,582 | | | | — | |
Dr. Ehlers | | $ | 16,200 | | | $ | 83,889 | | | | — | | | $ | 1,582 | | | | — | | | | — | | | | — | | | $ | 16,500 | | | $ | 168,428 | | | | — | | | $ | 1,582 | | | | — | |
Ms. Alexander | | $ | 16,200 | | | $ | 149,649 | | | $ | 10,690 | | | $ | 1,469 | | | | — | | | | — | | | | — | | | $ | 16,500 | | | $ | 176,553 | | | $ | 6,560 | | | $ | 1,527 | | | | — | |
Dr. McKenzie | | $ | 16,200 | | | $ | 51,107 | | | $ | 11,524 | | | $ | 1,213 | | | $ | 21,210 | | | | — | | | | — | | | $ | 16,500 | | | $ | 118,612 | | | $ | 5,179 | | | $ | 1,274 | | | $ | 12,841 | |
Mr. Covino | | $ | 16,200 | | | $ | 25,645 | | | | — | | | $ | 792 | | | | — | | | $ | 833 | | | | — | | |
Dr. Scangos | | $ | 3,604 | | | | — | | | | — | | | $ | 88 | | | | — | | | | — | | | $ | 7,252,104 | (16) | |
Mr. Clancy | | $ | 13,500 | | | $ | 151,179 | | | $ | 2,759 | | | $ | 923 | | | | — | | | | — | | | | — | | |
Mr. DiPietro | | $ | 9,818 | | | $ | 133,511 | | | | — | | | $ | 577 | | | | — | | | | — | | | $ | 2,043,163 | (17) | |
| (6) | Mr. Vounatsos joined Biogen as our Executive Vice President, Chief Commercial Officer effective April 18, 2016. Mr. Vounatsos was appointed our Chief Executive Officer and a member of our Board of Directors effective January 6, 2017. His base salary for 2017 was $1,100,000, of which he received a pro rata share from January 6, 2017 to December 31, 2017. From January 1, 2017 to January 5, 2017, he was paid at his prior rate of base salary ($750,000). |
| (7) | Mr. Capello was appointed as our Executive Vice President and Chief Financial Officer effective December 11, 2017. His base salary for 2017 was $750,000, of which he received a pro rata share from December 11, 2017 to December 31, 2017. Mr. Capello was not eligible to participate in our 2017 annual bonus plan and was not granted any stock awards in 2017. |
| (8) | Mr. Covino was appointed as our Interim Principal Financial Officer on July 1, 2017, and served in this role until December 11, 2017. |
(9) | Dr. Scangos ceased to be our Chief Executive Officer effective January 6, 2017. His base salary for 2017 was $1,500,000, of which he received a pro rata share from January 1, 2017 to January 6, 2017. Dr. Scangos was not granted any stock awards in 2017. |
(10) | Mr. Clancy ceased to be our Executive Vice President, Finance and Chief Financial Officer effective July 1, 2017. His base salary for 2017 was $890,586, of which he received a pro rata share from January 1, 2017 to July 1, 2017. Mr. Clancy was not eligible to receive a bonus under our 2017 annual bonus plan. |
(11) | Mr. DiPietro ceased to be our Executive Vice President, Human Resources effective May 26, 2017, and ceased to be employed by us effective September 30, 2017. His base salary for 2017 was $678,794, of which he received a pro rata share from January 1, 2017 to September 30, 2017. Mr. DiPietro was not eligible to receive a bonus under our 2017 annual bonus plan. |
(12) | The amount for Mr. Vounatsos includes a Company 401(k) match of $1,731 paid in 2017 for benefit year 2016 in connection with an administrative correction. |
(13) | Represents reimbursements of expenses relating to tax, financial and estate planning and executive physicals as described under the heading “Executive Physicals, Tax Preparation, Financial and Estate Planning” above. The amount for Ms. Alexander includes the |
| | | | |
52 | |  | |  |
| | |
5 | (9) | Executive Compensation Matters (continued)
|
| 2017 benefit of $5,720 and reimbursement during 2017 of the 2016 benefit of $4,970. The amount for Dr. McKenzie includes the 2017 benefit of $7,500 and reimbursement during 2017 of the 2016 benefit of $4,024. The amount for Mr. Clancy includes the reimbursement during 2017 of the 2016 benefit of $2,759. |
(14) | The amounts for Mr. Vounatsos and Dr. McKenzie reflectreflects relocation benefits under the Company’s Executive Relocation Policy and includeincludes a taxgross-upsgross-up of $25,889 and $2,672, respectively. |
(15) | The amount for Mr. Covino reflects an employee service recognition award and related $333 taxgross-up. |
(16) | On January 6, 2017, Dr. Scangos ceased to be our Chief Executive Officer and the Company paid him the severance benefits payable under his employment agreement, which consisted of (a) a lump sum cash payment in the amount of $7.2 million (two times his annual base salary and target annual bonus), (b) a prorated annual bonus payment for the period January 1, 2017 through January 6, 2017, based on actual Company performance and assuming individual performance of 100% and (c) continuation of certain subsidized medical, dental and vision benefits until July 1, 2018. The amount of the prorated annual bonus equaled $44,877 and the cost of the continuation of certain subsidized medical, dental and vision benefits equaled $7,227. In addition, Dr. Scangos was eligible to receive nine months of executive-level outplacement services at our cost equaling $28,000; however, Dr. Scangos did not utilize this benefit. |
(17) | On May 26, 2017, Mr. DiPietro ceased to be our Executive Vice President, Human Resources and ceased to be employed by us effective September 30, 2017. The Company provided him the severance benefits required under our executive severance policy for Executive Vice Presidents, which consisted of (a) a lump sum cash payment of $2,019,413 (21 months of base salary and target bonus) (b) continuation of certain subsidized medical, dental and vision benefits until the earlier of (1) January 31, 2019, or (2) the date on which he becomes eligible to receive benefits through another employer. In addition, our Compensation Committee agreed to permit continued vesting ofone-third of his outstanding LTI awards scheduled to vest on February 15, 2018, February 22, 2018, and February 23, 2018, as if he had remained employed by the Company through February 2018. The cost of the continuation of certain subsidized medical, dental and vision benefits equaled (assuming the benefits continue until January 31, 2019) $23,750. Mr. DiPietro was eligible to receive up to 12 months of executive-level outplacement services at our cost equaling $32,000; however, Mr. DiPietro did not utilize this benefit.$1,518. |
| | | | |
| 53 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
20172018 Grants of Plan-Based Awards
The following table shows additional information regarding all grants of plan-based awards made to our NEOs for the year ended December 31, 2017.2018.
| | | | | | | | Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) | | | | | | Estimated Future Payouts Under Equity Incentive Plan Awards (#)(1) | | | All Other Stock Awards: Number of Shares or Units (#)(i) | | Grant Date Fair Value of Stock Awards(2) (j) | | | | | | Estimated Future Payouts Under Non-Equity Incentive Plan
Awards(1) | | | | Estimated Future Payouts
Under Equity Incentive Plan
Awards(#)(1) | | All Other Stock Awards: Number of
Shares or Units
(#) (i) | | Grant Date Fair Value of Stock Awards(2) (j) |
Name (a) | | Grant Date (b) | | Notes | | Threshold (c) | | Target (d) | | Maximum (e) | | | | Threshold (f) | | Target (g) | | Maximum (h) | | | Grant Date (b) | | Notes | | Threshold (c) | | Target (d) | | Maximum (e) | | | | Threshold (f) | | Target (g) | | Maximum (h) |
Michel Vounatsos | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 6,375 | | | 12,750 | | | 25,500 | | | — | | $ | 4,868,786 | | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 9,080 | | | | 18,160 | | | | 36,320 | | | | — | | | $ | 6,856,251 | |
| | | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 8,543 | | | 17,085 | | | 34,170 | | | — | | $ | 4,999,754 | | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 6,646 | | | | 13,292 | | | | 26,584 | | | | — | | | $ | 4,208,646 | |
| | | 02/15/2017 | | | (5) | | $ | 343,750 | | | $ | 1,375,000 | | | $ | 3,093,750 | | | | | | — | | | | — | | | | — | | | — | | | — | | | | | 02/12/2018 | | | (5) | | $ | 455,000 | | | $ | 1,820,000 | | | $ | 4,095,000 | | | | | | — | | | | — | | | | — | | | | — | | | | — | |
Jeffrey D. Capello | | | — | | | — | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | — | | | — | | | | 01/02/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,245 | | | | 4,490 | | | | 8,980 | | | | — | | | $ | 1,789,136 | |
| | | | 01/02/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 1,646 | | | | 3,292 | | | | 6,584 | | | | — | | | $ | 1,100,088 | |
| | | | | 02/12/2018 | | | (5) | | $ | 131,250 | | | $ | 525,000 | | | $ | 1,181,250 | | | | | | — | | | | — | | | | — | | | | — | | | | — | |
Michael D. Ehlers | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 2,040 | | | 4,080 | | | 8,160 | | | — | | $ | 1,558,060 | | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,960 | | | | 5,920 | | | | 11,840 | | | | — | | | $ | 2,235,068 | |
| | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 2,733 | | | 5,465 | | | 10,930 | | | — | | $1,599,278 | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 2,169 | | | | 4,337 | | | | 8,674 | | | | — | | | $ | 1,373,224 | |
| | | 02/15/2017 | | | (5) | | $139,016 | | | $556,063 | | | $1,251,141 | | | | | | — | | | | — | | | | — | | | — | | | — | | | | 02/12/2018 | | | (5) | | $ | 145,966 | | | $ | 583,866 | | | $ | 1,313,698 | | | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | | | 02/12/2018 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | | 4,735 | | | $ | 1,499,243 | |
Susan H. Alexander | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 2,040 | | | 4,080 | | | 8,160 | | | — | | $ | 1,558,060 | | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,528 | | | | 5,055 | | | | 10,110 | | | | — | | | $ | 1,908,558 | |
| | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 1,848 | | | | 3,696 | | | | 7,392 | | | | — | | | $ | 1,170,264 | |
| | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 2,733 | | | 5,465 | | | 10,930 | | | — | | $1,599,278 | | | | 02/12/2018 | | | (5) | | $ | 131,106 | | | $ | 524,424 | | | $ | 1,179,954 | | | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | 02/15/2017 | | | (5) | | $126,672 | | | $506,689 | | | $1,140,051 | | | | | | — | | | | — | | | | — | | | — | | | — | | | | 02/12/2018 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | | 4,045 | | | $ | 1,280,768 | |
Paul F. McKenzie | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 1,753 | | | 3,505 | | | 7,010 | | | — | | $ | 1,338,443 | | | | 02/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | | | 2,368 | | | | 4,735 | | | | 9,470 | | | | — | | | $ | 1,787,683 | |
| | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 2,350 | | | 4,700 | | | 9,400 | | | — | | $1,375,408 | | | | 02/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | | | 1,731 | | | | 3,462 | | | | 6,924 | | | | — | | | $ | 1,096,173 | |
| | 02/15/2017 | | | (5) | | $105,656 | | | $422,625 | | | $950,906 | | | | | | — | | | | — | | | | — | | | — | | | — | | | | 02/12/2018 | | | (5) | | $ | 110,939 | | | $ | 443,757 | | | $ | 998,452 | | | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | | 03/01/2017 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | 2,730 | | $ | 800,600 | | | | 02/12/2018 | | | (6) | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | | 3,790 | | | $ | 1,200,028 | |
Gregory F. Covino | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 193 | | | 385 | | | 770 | | | — | | $ | 147,040 | | |
| | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 258 | | | 515 | | | 1,030 | | | — | | $ | 150,710 | | |
| | | 02/15/2017 | | | (5) | | $33,825 | | | $135,301 | | | $304,427 | | | | | | — | | | | — | | | | — | | | — | | | — | | |
George A. Scangos | | | — | | | — | | | — | | | | — | | | | — | | | | | | — | | | | — | | | | — | | | — | | | — | | |
Paul J. Clancy | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 1,913 | | | 3,825 | | | 7,650 | | | — | | $ | 1,460,686 | | |
| | | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 2,565 | | | 5,130 | | | 10,260 | | | — | | $ | 1,501,243 | | |
Kenneth A. DiPietro(7) | | 02/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | | 1,595 | | | 3,190 | | | 6,380 | | | — | | $1,218,157 | | |
| | | 02/15/2017 | | | (4) | | | — | | | | — | | | | — | | | | | 2,135 | | | 4,270 | | | 8,540 | | | — | | $ | 1,249,573 | | |
Notes to the 20172018 Grants of Plan-Based Awards Table
| (1) | Reflects the potential future payouts of awards granted in 20172018 under our 20172018 annual bonus plan and our LTI program for each NEO as of the respective grant dates. |
| (2) | Represents the grant date fair value of CSPUs,PSUs, MSUs and RSUs, computed in accordance with ASC 718, excluding the effect of estimated forfeitures. The grant date fair value for MSU awards areis estimated as of the date of grant using a lattice model with a Monte Carlo simulation based on the probable outcome of applicable performance conditions. Assumptions used in this calculation are included on pageF-46 in footnote 16 of our 2017 Annual Report on Form10-K.The grant date fair value for both CSPUPSU and RSU awards wasis determined by multiplying the number of shares subject to the award (assuming target performance for CSPUs)PSUs) by the closing price of the Company’sour common stock on the grant date. For the 2018 Cash-Settled PSUs, the grant date value ofone-third of the award is included, which is the tranche of the award for which goals had been set relating to the 2018 performance period and fair value was determinable in 2018. The remaining tranches of the 2018 Cash-Settled PSUs will be included in compensation tables for 2019 and 2020, the years when goals will be set with respect to such performance periods and fair value is determinable. The assumptions used to calculate the grant date fair value of stock awards are included in footnote 16 of our 2018 Annual Report on Form10-K. The maximum payouts for these awards are included in the footnotes following the Summary Compensation Table above. |
| (3) | These amounts relate to the annual grant of MSUs. MSU grants represent 50% of the annual LTI grant date target value (excludingone-time RSU grants). These are performance-based RSUs tied to the growth in our stock price between the grant date and each of three annual vesting dates. The number of MSUs earned is determined on each vesting date. Columns (f), (g) and (h) represent the number of MSUs that can be earned based on performance at the threshold level of 50%, target level of 100% and the maximum level of 200%, respectively. To the extent earned, the award becomes eligible to vest ratably over three years, generally subject to continued service, as described in further detail under the heading “Long-Term Incentives (LTI)”Incentives” above. |
| (4) | These amounts relate to the annual grant of CSPUs. These are performance-based RSUs tied to our 2017 financial performance and subsequently subject to time-basedPSUs. PSU grants represent 50% of the annual LTI grant date target value (excludingone-time RSU grants). The PSUs have three-year cliff vesting. The numberamounts shown include the 2018 Stock-Settled PSUs andone-third of CSPUs earnedthe 2018 Cash-Settled PSUs, which is determinedthe tranche of the award for which goals had been set in early 2018 based on 2017 revenuesrelating to the 2018 performance period and adjusted free cash flowfair value was determinable in 2018. The remaining tranches of the 2018 Cash-Settled PSUs will be included in compensation tables for 2019 and 2020, the years when goals will be set with respect to such performance against target. CSPUs, if earned, will vest ratably over three years, generally subject to continued service. These awards are settled in cash or stock in the discretion of our Compensation Committee upon the vesting date based on the30-day average closing price of our common stock.periods and fair value is determinable. Columns (f), (g) and (h) represent the number of CSPUs2018 Stock-Settled PSUs and the 2018 tranche of the 2018 Cash-Settled PSUs that can be earned if the CSPU multiplierCompany Multiplier were 50%, 100% and 200%, respectively. For additional information on our PSU awards, please see “Long-Term Incentives—2018 PSUs” above. |
| (5) | These amounts relate to our 20172018 annual bonus plan. The amounts shown in column (d) represent the 20172018 target payout amount based on the target percentage applied to each NEO’s annual base salary as of December 31, 2017.2018. For 2017,2018 the bonus targets were 125%140% of base salary for Mr. Vounatsos 35% of base salary for Mr. Covino and 70% of base salary for all other current NEOs. Dr. Scangos and Messrs. Clancy and DiPietro were not eligible to participate in our 2017 annual bonus plan. The amounts in column (c), (d) and (e) represent a payment if the Company Multiplier and the Individual Multiplier were each 50%, 100% and 150%, respectively. Actual amounts paid to each NEO |
| | | | |
| 54 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
| | respectively. Actual amounts paid to each NEO under our 20172018 annual bonus plan are included in the“Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table above. |
| (6) | These amounts relate to aone-time granttransition awards of time-based RSUs for Dr. McKenzie,granted to each of the NEOs, except Messrs. Vounatsos and Capello, as described in further detail in the CD&A above under the heading “2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Arrangement with Dr. McKenzie.” |
(7) | As a result of his separation from the Company on September 30, 2017, our Compensation Committee agreed to permit continued vesting of“2018one-thirdOne-Time of Mr. DiPietro’s outstanding LTI awards scheduled to vest on February 15, 2018, February 22, 2018, and February 23, 2018.Transition Awards” above. |
Outstanding Equity Awards at 20172018 FiscalYear-End
The following table summarizes the equity awards that were outstanding as of December 31, 2017,2018, for each of our NEOs.
| | | | | | | | Option Awards | | Stock Awards | |
| | | | | | Option Awards(1) | | Stock Awards | | | | | | | | | | | | | | | | | Equity Incentive Plan
Awards | |
| | | | | | | | | | | | | | | | | Equity Incentive Plan
Awards | | | | | |
| | | Grant
Date (b) | | | Number of Securities
Underlying Unexercised
Options | | Option
Exercise
Price (e) | | | Option Expiration Date (f) | | | Number of Shares or Units of Stock That Have Not Vested(2) (g) | | | Market Value of Shares or Units of Stock That Have Not Vested(3) (h) | | | Number of Unearned Shares or Units That Have Not Vested(4) (i) | | | Market Value of Unearned Shares or Units That Have Not Vested(3) (j) | | | Grant
Date (b) | | Notes | | Number of Securities Underlying Unexercised
Options | | | Option Exercise Price (e) | | Option Expiration Date (f) | | Number of Shares or Units of Stock That Have Not Vested (g) | | Market Value of Shares or Units of Stock That Have Not Vested (h) | | Number of Unearned Shares or Units That Have Not Vested (i) | | Market Value of Unearned Shares or Units That Have Not Vested(1) (j) | |
| (a) | | Exercisable (c) | | Unexercisable (d) | | | Exercisable (c) | | Unexercisable (d) | |
| Michel Vounatsos | | 5/2/2016 | | | | — | | | | — | | | | — | | | | — | | | 4,638 | | | $ | 1,477,528 | | | | — | | | | — | | | | 5/2/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 2,320 | | | $ | 698,134 | | | | — | | | | — | |
| | 5/2/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 6,496 | | | $ | 2,069,431 | | | | 5/2/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,252 | | | $ | 978,592 | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 21,015 | | | $ | 6,694,749 | | | | — | | | | — | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 14,080 | | | $ | 4,236,954 | | | | — | | | | — | |
| | | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 25,500 | | | $ | 8,123,535 | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 17,002 | | | $ | 5,116,242 | |
| | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 36,320 | | | $ | 10,929,414 | |
| | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 4,602 | | | $ | 1,384,834 | | | | 15,763 | | | $ | 4,743,402 | |
Jeffrey D. Capello | | | | 1/2/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,490 | | | $ | 1,351,131 | |
| | | | | 1/2/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,146 | | | $ | 344,854 | | | | 3,893 | | | $ | 1,171,482 | |
| Michael D. Ehlers | | 6/1/2016 | | | | — | | | | — | | | | — | | | | — | | | 3,588 | | | $ | 1,143,029 | | | | — | | | | — | | | | 6/1/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 1,821 | | | $ | 547,975 | | | | — | | | | — | |
| | | | 6/1/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,502 | | | $ | 752,902 | |
| | | | 6/1/2016 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 1,036 | | | $ | 311,753 | | | | — | | | | — | |
| | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 4,504 | | | $ | 1,355,344 | | | | — | | | | — | |
| | 6/1/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 4,996 | | | $ | 1,591,576 | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,442 | | | $ | 1,637,607 | |
| | 6/1/2016 | | | | — | | | | — | | | | — | | | | — | | | 2,070 | | | $ | 659,440 | | | | — | | | | — | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 11,840 | | | $ | 3,562,893 | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 6,722 | | | $ | 2,141,428 | | | | — | | | | — | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,501 | | | $ | 451,681 | | | | 5,143 | | | $ | 1,547,632 | |
| | | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 8,160 | | | $ | 2,599,531 | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 4,735 | | | $ | 1,424,856 | | | | — | | | | — | |
| Susan H. Alexander | | 2/24/2009 | | | 6,395 | | | | — | | | $ | 48.52 | | | 2/23/2019 | | | | — | | | | — | | | | — | | | | — | | | | 2/24/2009 | | | (6) | | | 6,395 | | | | — | | | | $48.52 | | | | 2/23/2019 | | | | — | | | | — | | | | — | | | | — | |
| | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | 937 | | | $ | 298,500 | | | | — | | | | — | | | | 2/22/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 1,806 | | | $ | 543,462 | | | | — | | | | — | |
| | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 974 | | | $ | 310,287 | | | | 2/22/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 2,482 | | | $ | 746,883 | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | 3,559 | | | $ | 1,133,791 | | | | — | | | | — | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 4,504 | | | $ | 1,355,344 | | | | — | | | | — | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 4,960 | | | $ | 1,580,107 | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,442 | | | $ | 1,637,607 | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 6,722 | | | $ | 2,141,428 | | | | — | | | | — | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,110 | | | $ | 3,042,301 | |
| | | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 8,160 | | | $ | 2,599,531 | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,279 | | | $ | 384,877 | | | | 4,384 | | | $ | 1,319,233 | |
| | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 4,045 | | | $ | 1,217,221 | | | | — | | | | — | |
| Paul F. McKenzie | | 3/1/2016 | | | | — | | | | — | | | | — | | | | — | | | 1,214 | | | $ | 386,744 | | | | — | | | | — | | | | 3/1/2016 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 616 | | | $ | 185,367 | | | | — | | | | — | |
| | 3/1/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 1,692 | | | $ | 539,020 | | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 5,781 | | | $ | 1,841,653 | | | | — | | | | — | | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 7,010 | | | $ | 2,233,176 | | |
| | | 3/1/2017 | | | | — | | | | — | | | | — | | | | — | | | 2,730 | | | $ | 869,696 | | | | — | | | | — | | |
| Gregory F. Covino | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | 99 | | | $ | 31,538 | | | | — | | | | — | | |
| | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 104 | | | $ | 33,131 | | | | 3/1/2016 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 848 | | | $ | 255,180 | |
| | 12/1/2015 | | | | — | | | | — | | | | — | | | | — | | | 165 | | | $ | 52,564 | | | | — | | | | — | | | | 2/15/2017 | | | (2) | | | — | | | | — | | | | — | | | | — | | | | 3,875 | | | $ | 1,166,065 | | | | — | | | | — | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | 425 | | | $ | 135,392 | | | | — | | | | — | | | | 2/15/2017 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 4,674 | | | $ | 1,406,500 | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 596 | | | $ | 189,868 | | | | 3/1/2017 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 1,820 | | | $ | 547,674 | | | | — | | | | — | |
| | 12/1/2016 | | | | — | | | | — | | | | — | | | | — | | | 634 | | | $ | 201,973 | | | | — | | | | — | | | | 2/12/2018 | | | (3) | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 9,470 | | | $ | 2,849,712 | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 633 | | | $ | 201,655 | | | | — | | | | — | | | | 2/12/2018 | | | (4) | | | — | | | | — | | | | — | | | | — | | | | 1,194 | | | $ | 359,298 | | | | 4,108 | | | $ | 1,236,179 | |
| | | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 770 | | | $ | 245,299 | | | | 2/12/2018 | | | (5) | | | — | | | | — | | | | — | | | | — | | | | 3,790 | | | $ | 1,140,487 | | | | — | | | | — | |
| George A. Scangos | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | 4,361 | | | $ | 1,389,284 | | | | — | | | | — | | |
| | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 4,530 | | | $ | 1,443,122 | | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | 19,808 | | | $ | 6,310,235 | | | | — | | | | — | | |
| | | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 27,604 | | | $ | 8,793,806 | | |
| Paul J. Clancy | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | 852 | | | $ | 271,422 | | | | — | | | | — | | |
| | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 887 | | | $ | 282,572 | | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | 5,418 | | | $ | 1,726,012 | | | | — | | | | — | | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 7,546 | | | $ | 2,403,929 | | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 6,310 | | | $ | 2,010,177 | | | | — | | | | — | | |
| | | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 7,650 | | | $ | 2,437,061 | | |
| Kenneth A. DiPietro | | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | 312 | | | $ | 99,394 | | | | — | | | | — | | |
| | 2/23/2015 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 325 | | | $ | 103,535 | | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | 635 | | | $ | 202,292 | | | | — | | | | — | | |
| | 2/22/2016 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 898 | | | $ | 286,076 | | |
| | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | 578 | | | $ | 184,133 | | | | — | | | | — | | |
| | | 2/15/2017 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | 708 | | | $ | 225,548 | | |
Notes to the Outstanding Equity Awards at 20172018 Fiscal Year End Table
| (1) | AllThe market value of awards is based on the closing price of our stock optionson December 31, 2018 ($300.92), as reported by Nasdaq.
|
| (2) | CSPUs were granted with aten-year term. Stock options vest 25%in 2017 and 2016. The numbers in column (g) reflect the number of CSPUs that were earned based on our financial performance for each of 2017 and 2016, but that had not vested based on our service-based vesting requirement as of December 31, 2018. CSPUs that have been earned based on the first four anniversariessatisfaction of the performance conditions vest ratably over three years from the grant date. It has not beenThe cash payout for these awards will be based on our30-day average closing stock price at vesting. Grants made before February 1, 2017, were adjusted pursuant to the Company’s practiceanti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
| (3) | MSUs were granted in 2018, 2017 and 2016. These are performance-based RSUs earned based on the growth in our stock price between the dates of grant and vesting. Earned MSUs are eligible to cash out stock options having an exercise price greater thanvest ratably over three years. The number and value shown in columns (i) and (j), respectively, reflects maximum performance results for MSUs granted in 2018, 2017 and 2016 based on the market price (i.e., underwaterestimated performance atyear-end in each case except for Mr. Capello’s January 2, 2018, grant, for which columns (i) and (j) reflect target performance results based on the estimated performance atyear-end. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
| | | | |
| 55 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
| (4) | options). These stock options were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
(2) | CSPUsPSUs were granted in 2017, 20162018. The PSUs are subject to three-year cliff vesting. In addition, 60% of the PSUs (based on the grant date target value) will be settled in shares of our common stock and 2015.performance will be based upon achievement of cumulative three-year financial and pipeline goals. The numbersremaining 40% of the PSUs will be settled in columncash and performance will be based upon the achievement of three annual financial goals determined at the beginning of each relevant year. The number and value shown in columns (g) and (h), respectively, reflect the number of CSPUs2018 Cash-Settled PSUs that were earned based on our financialachievement of the annual performance goals for the 2018 tranche, but that will not vest until February 12, 2021. The number and value shown in columns (i) and (j), respectively, reflect the remaining portion of the PSUs granted in 2018 (including the 2019 and 2020 tranches of the 2018 Cash-Settled PSUs) assuming target performance results. For additional information on our PSU awards, please see “Long-Term Incentives—2018 PSUs” above. |
| (5) | RSUs were granted in 2018, 2017 and 2016 for special circumstances. 2018 RSU awards relate toone-time transition awards granted to each of 2017, 2016the NEOs, except Messrs. Vounatsos and 2015, but that had not vested based on our service-based vesting requirementCapello, as of December 31, 2017. CSPUs that have been earned based on the satisfaction of the performance conditions vest ratably over three years from the grant date. The cash payout for these awards will be based on ourdescribed under “201830-dayOne-Time average closing stock price at vesting.Transition Awards” above. For Dr. Ehlers and Dr. McKenzie, and Mr. Covino, the amounts in this column also reflect 3,104 RSUs granted to Dr. Ehlers on June 1, 2016, in connection with his hiring and 2,730 RSUs granted to Dr. McKenzie on March 1, 2017, under hisone-time award, as described in more detail under the heading “2017“Executive Compensation Matters—Compensation Discussion and Analysis—2017 and 2018 Hiring- and Transition-Related Compensation Decisions—ArrangementsArrangement with Dr. McKenzie” above and 494 and 951 RSUs granted to Mr. Covino under his special recognition awards on December 1, 2015 and December 1, 2016, respectively,in our 2018 proxy statement, in each case vesting ratably over three years from the grant date. Grants made before February 1, 2017, were adjusted pursuant to the anti-dilution provisions of such awards in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above reflect adjusted share amounts. |
(3)(6) | The market value of awards is based on the closing price of ourThese stock on December 29, 2017 ($318.57), as reported by Nasdaq. |
(4) | MSUsoptions were granted in 2017, 2016 and 2015.with aten-year term. Stock options vest 25% on each of the first four anniversaries of the grant date. These are performance-based RSUs earned based on the growth in our stock price between the dates of grant and vesting. Earned MSUs are eligible to vest ratably over three years. The number and value shown in columns (i) and (j), respectively, reflects maximum performance results for MSUs granted in 2016 and 2017 and target performance results for MSUs granted in 2015 based on the prior year’s performance in each case. Grants made before February 1, 2017,options were subsequently adjusted pursuant to the anti-dilution provisions of such awardsthe award in connection with thespin-off of our hemophilia business on February 1, 2017. The amounts listed above in column (i) reflect adjusted share and exercise price amounts. |
20172018 Option Exercises and Stock Vested
Our executive officers must usepre-established trading plans to sell shares of Biogenour common stock. Trading plans may only be entered into during an open trading window and when the executive is not in possession of materialnon-public information about the Company, and we require a waiting period following the establishment of a trading plan before any trades may be executed. Our policy is designed to provide safeguards that will allowwhile allowing our executives an opportunity to realize the value intended by the Company in granting equity-based LTI awards.
Our NEOs are also subject to the stock ownership guidelines described above under the heading “Stock Ownership Guidelines.”
The following table shows information regarding vesting of stock awards for each NEO during the year ended December 31, 2017.2018. None of the NEOs exercised stock options during the year ended December 31, 2017.2018.
| | | | | Stock Awards | | | Stock Awards |
| Name | | Number of Shares Acquired on Vesting(1) | | | | | | Value Realized on Vesting(2)(3) | | | Number of Shares
Acquired on
Vesting(1) | | | | Value
Realized on
Vesting(2)(3) |
Michel Vounatsos | | | 4,054 | | | | | $ | 1,103,874 | | | | 16,294 | | | $ | 5,013,149 | |
Jeffrey D. Capello | | | — | | | | | | — | | | | — | | | | — | |
Michael D. Ehlers | | | 4,059 | | | | | $ | 1,029,660 | | | | 8,115 | | | $ | 2,471,489 | |
Susan H. Alexander | | | 10,994 | | | | | $ | 3,006,369 | | | | 9,111 | | | $ | 2,844,100 | |
Paul F. McKenzie | | | 1,078 | | | | | $ | 307,318 | | | | 5,419 | | | $ | 1,672,605 | |
Gregory F. Covino | | | 1,734 | | | | | $ | 496,773 | | |
George A. Scangos | | | 49,837 | | | | | $ | 13,660,677 | | |
Paul J. Clancy | | | 12,816 | | | | | $ | 3,515,428 | | |
Kenneth A. DiPietro | | | 11,097 | | | | | $ | 3,037,824 | | |
Notes to the 20172018 Option Exercises and Stock Vested Table
| (1) | CSPUs were settled in cash for all of our NEOs. The number of actual shares of our common stock acquired on vesting with respect to MSUs and RSUs, as applicable, after shares were withheld to pay the minimum withholding of taxes was as follows: |
| | |
| |
| | Net Shares Acquired(4) |
Mr. Vounatsos | | 4,414 |
Mr. Capello | | — |
Dr. Ehlers | | 2,545 |
Ms. Alexander | | 2,586 |
Dr. McKenzie | | 1,830 |
| | | | |
| 56 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
(1) | With the exception of Dr. Scangos’ 2014 CSPUs, CSPUs were settled in cash for all of our NEOs. The number of actual shares of our common stock acquired on vesting after shares were withheld to pay the minimum withholding of taxes was as follows: |
| | |
| | Net Shares
Acquired(4)
|
Mr. Vounatsos
| | 1,172 |
Mr. Capello
| | — |
Dr. Ehlers
| | 1,546 |
Ms. Alexander
| | 3,150 |
Dr. McKenzie
| | 324 |
Mr. Covino
| | 916 |
Dr. Scangos
| | 18,890 |
Mr. Clancy
| | 3,726 |
Mr. DiPietro
| | 3,240 |
| (2) | The value realized for MSUs and RSUs are calculated by multiplying the closing price of a share of our common stock on the vesting date by the total number of shares that vested on such date. The value realized for CSPUs is calculated using the30-day average closing price of the common stock of the Company through the vesting date. |
| (3) | The value realized upon vesting for Mr. CovinoDr. McKenzie includes CSPUs with a value of $141,937 and MSUs with a value of $118,422,$304,487, the receipt of which was deferred under the SSP, as described below. Terms of thenon-qualified deferred compensation plan are described under the heading “2017“2018Non-Qualified Deferred Compensation” below. |
| (4) | MSUs and RSUs were settled in shares of our common stock. CSPUs were settled in cash for all of our NEOs, other than Dr. Scangos, who had a portion of his CSPUs settled in shares of our common stock. For Dr. Scangos, in 2015, our Compensation Committee exercised its discretion to settle Dr. Scangos’ 2014 CSPUs in shares of our common stock; the net shares acquired by Dr. Scangos reflected in the table above represent 13,447 MSUs and 5,533 CSPUs settled in shares.cash. |
20172018Non-Qualified Deferred Compensation
The SSP covers our executive officers and other eligible employees in the U.S. whose base salary and annual cash incentives for the year exceed a specified Internal Revenue Services limit ($270,000275,000 in 2017)2018) receive a Company-paid restoration match on the portion of their base salary, annual bonus and cash payments in respect of CSPUs and PSUs, as applicable, that exceeds this limit; the restoration match equals 6% of this excess compensation. The restoration match feature is intended to provide the amount of matching employer contributions that the participant would otherwise have been eligible to receive under our 401(k) plan but for the $270,000$275,000 limit imposed by Section 401(a)(17) of the Internal Revenue Code. In addition, eligible employees may make voluntary contributions of up to 80% of their base salary and 100% of their annual bonus and cash payments in respect of CSPUs and PSUs, as applicable, to the SSP, and thereby defer income taxes on such amounts until distribution is made from the SSP. The Company does not match participants’ voluntary contributions to the SSP. The SSP provides for immediate vesting of the restoration match consistent with our immediate vesting of the Company match provided under our 401(k) plan.
Notional SSP accounts are maintained for each participant. Accounts include employee and employer contributions and reflect the performance of notional investments selected by the employee or a default investment if the employee does not make a selection. These notional investment options
include the mutual funds offered under our 401(k) plan as well as a fixed rate option which earns a rate of return determined each year by the Company’s retirement committee. For contributions to the SSP fixed rate option in 2017,2018, this rate of return iswas set at 5%. Contributions to the fixed rate option continue to earn interest at the rate of return that was in effect during the year of contribution. The excess of the interest rate paid on the fixed rate option above 120% of the applicable federal long-term rate (compounded quarterly) earned by our NEOs during 20172018 is shown in the Summary Compensation Table. We fund the SSP liabilities through corporate ownedcorporate-owned life insurance (COLI), which we purchase with the written consent of SSP participants, and investments in mutual funds. We believe that the COLI policies and mutual funds will be sufficient to cover plan liabilities through the projected payout date so the plan will not require direct funding by the Company. Upon enrollment in the SSP, a participant must elect when and how distributions will be made from the participant’s account. Distributions can be made upon termination of the participant’s employment, either in a lump sum or up to 15 annual installments, or at a specified future date while the participant is still employed (an“in-service” distribution), either in a lump sum or up to 5 annual installments. Further, upon enrollment, a participant must also elect a distribution method upon death or a change in control of the Company, which can either be a lump sum payment or, if different, the method selected for payment upon termination of employment.
| | | | |
57 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued) |
The following table shows a summary of all contributions to, earnings on and distributions received from the SSP for each of our NEOs for the year ended December 31, 2017.2018. The account balances as ofyear-end include all contributions and interest amounts earned by our NEOs through the end of 20172018 plus the SSP contributions that the Company made in early 20182019 based on earnings in the last quarter of 2017.2018.
| | | Name | | Executive Contributions in Last Fiscal Year(1) | | | Company Contributions in Last Fiscal Year(2) | | | Aggregate Earnings in Last Fiscal Year(3) | | | Aggregate Distributions in Last Fiscal Year | | | Aggregate Balance at Last Fiscal Year-End(4) | | | Executive
Contributions
in Last Fiscal
Year(1) | | | Company
Contributions
in Last Fiscal
Year(2) | | | Aggregate
Earnings
in Last
Fiscal
Year(3) | | Aggregate
Distributions
in Last
Fiscal Year | | Aggregate
Balance at
Last Fiscal Year-End(4) | |
Michel Vounatsos | | $ | 1,025,867 | | | $ | 113,911 | | | $ | 51,551 | | | | — | | | $ | 1,513,091 | | | $ | 3,071,852 | | | $ | 387,134 | | | $ | 205,737 | | | — | | $ | 5,160,044 | |
Jeffrey D. Capello | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | $ | 28,500 | | | $ | 60 | | | — | | $ | 28,560 | |
Michael D. Ehlers | | $ | 365,160 | | | $ | 83,889 | | | $ | 61,885 | | | | — | | | $ | 643,125 | | | $ | 552,463 | | | $ | 168,428 | | | $ | (80,992 | ) | | — | | $ | 1,270,192 | |
Susan H. Alexander | | $ | 573,897 | | | $ | 149,649 | | | $ | 324,632 | | | | — | | | $ | 5,875,553 | | | $ | 826,451 | | | $ | 176,553 | | | $ | 392,167 | | | — | | $ | 7,259,032 | |
Paul F. McKenzie | | $ | 221,128 | | | $ | 51,107 | | | $ | 16,457 | | | | — | | | $ | 348,429 | | | $ | 860,425 | | | $ | 118,612 | | | $ | 2,629 | | | — | | $ | 1,320,342 | |
Gregory F. Covino | | $ | 260,359 | | | $ | 25,645 | | | $ | 143,953 | | | | — | | | $ | 1,564,049 | | |
George A. Scangos | | $ | 768,306 | | | | — | | | $ | 1,192,859 | | | $ | 4,414,508 | | | $ | 9,166,069 | | |
Paul J. Clancy | | $ | 68,842 | | | $ | 151,179 | | | $ | 126,811 | | | | — | | | $ | 2,198,361 | | |
Kenneth A. DiPietro | | | — | | | $ | 133,511 | | | $ | 22,997 | | | $ | 887,238 | | | $ | 20,698 | | |
| | | | |
| 57 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Notes to the 20172018Non-Qualified Deferred Compensation Table
| (1) | The amounts in this column are also included, in part, in columns (c), (e) and/or (f) of the Summary Compensation Table asnon-qualifiedand represent deferral of salary,non-qualified deferral of payments under our 20172018 annual bonus plan andnon-qualified deferral of CSPU payments, respectively. Thenon-qualified deferral of MSU vested shares is only applicable to Mr. Covino, based on hispre-2015 election. Historically, eligible employees were allowed to make voluntary contributions of up to 100% of their MSU vested shares. Effective as of January 1, 2015, deferral of vested shares under MSU awards granted on or after January 1, 2015, is no longer permitted under the SSP. |
| (2) | The amounts in this column are also included in column (h) of the Summary Compensation Table for 20172018 as Company contributions to the SSP. |
| (3) | Earnings in excess of 120% of the applicable federal long-term rate are reported in column (g) of the Summary Compensation Table for 20172018 for Mr. Vounatsos ($18,881)80,663), Dr. Ehlers ($1,799)5,258), Ms. Alexander ($148,961),188,056) and Dr. McKenzie ($2,471), Mr. Covino ($22,787), Dr. Scangos ($159,968), Mr. Clancy ($61,983) and Mr. DiPietro ($149)12,367). |
| (4) | The following table lists the compensation deferrals during 20162018 and 20152017 by the NEOs, as reported, where applicable, in the proxy statement for our 20172018 and 20162017 Annual Meetings of Stockholders: |
| | | | | | | | | Amounts Previously
Reported as Deferred | |
| | | Amounts Previously
Reported as Deferred | | |
| Name | | 2016 | | | 2015 | | | 2017 | | | 2016 | |
Mr. Vounatsos | | $ | 300,000 | | | | — | | | $ | 1,025,867 | | | $ | 300,000 | |
Mr. Capello | | | | — | | | | — | |
Dr. Ehlers | | $ | 116,250 | | | | — | | | $ | 365,160 | | | $ | 116,250 | |
Ms. Alexander | | $ | 259,816 | | | $ | 733,687 | | | $ | 573,897 | | | $ | 259,816 | |
Dr. Scangos | | $ | 504,375 | | | $ | 1,368,040 | | |
Mr. DiPietro | | | — | | | | — | | |
Dr. McKenzie | | | $ | 221,128 | | | | — | |
| | This column also includes Company contributions and compensation earned and deferred in prior years, which was disclosed in our prior proxy statements where applicable, together with earnings on these amounts. |
| | | | |
58 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued) |
Potential Payments Upon Termination or Change in Control
Executive Severance Policy
Definition of Key Terms Relating to our Executive Severance Policy
Our executive severance policy and benefits refer to certain key terms. These terms are defined in our 2017 Omnibus Equity Plan.
Executive Vice President Arrangements
Each of our current NEOs, other than Messrs.Mr. Vounatsos, and Covino, were covered by our executive severance policy in 20172018 under which they were eligible to receive the following benefits if certain events occurred during 2017:2018:
In the event of a termination of employment other than for cause and other than by reason of the executive’s death or disability, the NEO would be entitled to receive a lump sum severance payment equal to a minimum of 12 months of such NEO’s then base salary and target bonus, with an additional 2 months of base salary and target bonus for each full year of service to a maximum benefit of 21 months of base salary and target bonus. We refer to the number of months of severance a NEO is entitled to receive as the “severance period.”
If, within two years following a corporate transaction or a corporate change in control, the NEO experiences a termination of employment other than for cause orand other than by reason of death or disability or experiences an involuntary employment action, the NEO would be entitled to a lump sum severance payment equal to two times the NEO’s then- annual base salary plus target annual bonus. These payments are in lieu of any payment in the preceding paragraph.
| | an involuntary employment action, the NEO would be entitled to a lump sum severance payment equal to two times the NEO’s then-annual base salary plus target annual bonus. These payments are in lieu of any payment in the preceding paragraph. |
The payment of these severance benefits is conditioned upon execution of an irrevocable release in favor of the Company.
The executive severance policy does not pay severance upon a termination for cause, voluntary resignation, retirement or death or disability.
In any case where severance is payable under our executive severance policy, our NEOs would also receive continuation of medical, dental and vision insurance benefits until the earlier of the last date of the severance period or the date the executive becomes eligible to participate in another employer’s medical, dental and vision insurance plans. NEOs would also be provided up to 12 months of executive-level outplacement services at our cost.
Vice President Arrangements
Mr. Covino participated in our vice president severance policy in 2017 under which he was eligible to receive the following benefits if certain events occurred during 2017:
In the event of a termination of employment other than for cause and other than by reason of Mr. Covino’s death or disability, he would be entitled to receive a lump sum severance payment equal to a minimum of 6 months of his then base salary and target bonus, with an additional 1 month of base salary and target bonus for each full year of service to a maximum benefit of 12 months of base salary and target bonus. We refer to the number of months of severance Mr. Covino is entitled to as the “vice president severance period.”
If, within two years following a corporate transaction or a corporate change in control, Mr. Covino experiences a termination of employment other than for cause or by reason of death or disability or experiences an involuntary employment action, Mr. Covino would be entitled to a lump sum severance payment equal to one times his then-annual base salary plus target annual bonus. These payments are in lieu of any payment in the preceding paragraph.
The payment of these severance benefits is conditioned upon execution of an irrevocable release in favor of the Company. The vice president severance policy does not pay severance upon a termination for cause, voluntary retirement or death or disability.
In any case where severance is payable under our vice president severance policy, Mr. Covino would also receive continuation of medical, dental and vision insurance benefits until the earlier of the last date of the vice president severance period or the date he becomes eligible to participate in another employer’s medical, dental and vision insurance plans. Mr. Covino would also be provided up to six months of executive-level outplacement services at our cost.
Annual Bonus Plan
Our annual bonus plan provides for a prorated target bonus payment upon a termination of employment due to the death or disability of the participant. As our annual bonus plan provides for payment of a full bonus to any participant remaining employed on the last day of the plan year, annual bonus amounts are not included in the Potential Post-TerminationPost- Termination Payments Table below.
| | | | |
| 58 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Mr. Vounatsos’ Arrangements
We entered into an employment agreement with Mr. Vounatsos effective January 6, 2017, with an initial term
| | | | |
59 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued) |
ending on December 31, 2019, at which time the term automatically extends for additional12-month periods until the agreement is otherwise terminated in accordance with its terms.
Under Mr. Vounatsos’ employment agreement, if his employment is terminated by the Company without cause or if he terminates his employment for good reason (referred to in his employment agreement as an involuntary employment action), then he would be entitled to a lump sum payment of cash severance in the amount of one andone-half times his annual base salary and target annual bonus. If, however, such termination of employment occurs within two years following a corporate transaction or a corporate change in control (CIC), then he would be entitled to a lump sum payment of cash severance in the amount of two times his annual base salary and target annual bonus. Mr. Vounatsos would also receive continuation of medical, dental and vision benefits until the earlier of 18 months (24 months if within 2 years of a CIC) following the date his employment terminates or the date upon which he becomes eligible to receive substantially comparable benefits through another employer. In addition, he would be entitled to receive a pro rata portion of his annual cash bonus for the year that such termination occurs based on actual performance or, in the event the termination occurs within two years following a CIC, thehis target annual bonus. Mr. Vounatsos would also be provided executive-level outplacement services for a12-month period following the termination date at our cost. The payment of Mr. Vounatsos’ severance benefits is conditioned upon execution of general release in favor of the Company.
During 2017, Mr. Vounatsos invested $1.0 million in Biogen common stock.
Dr. Ehlers’ Additional Arrangements
In connection with the hiring of Dr. Ehlers, we agreed that if a new CEO were appointed within two years from his start date (i.e., prior to May 9, 2018) and as a result of the appointment (1) Dr. Ehlers’ employment is terminated other than for cause or (2) if Dr. Ehlers terminates his employment, following a notice and cure period, because there is a material and substantial change in the Company’s financial spend committed to the Research and Development organization or a material alteration and diminution in Dr. Ehlers’ authority, duties or responsibilities, then he would be entitled to receive severance benefits and accelerated vesting of outstanding LTI awards as if a change in control and involuntary employment action had occurred under our executive severance policy and our 2008 Omnibus Equity Plan.
Dr. Scangos’ Arrangements
On January 6, 2017, Dr. Scangos ceased to be our Chief Executive Officer and the Company paid him the severance benefits payable under his employment agreement, as described in the CD&A above under the heading “2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Dr. Scangos’ Arrangements.”
Mr. Clancy’s Arrangements
Mr. Clancy voluntarily separated from the Company on July 1, 2017, and did not receive any severance benefits in connection with his separation. Mr. Clancy’s outstanding LTI awards were eligible for retirement vesting with either accelerated or continued vesting, as applicable, pursuant to the retirement provision under our 2008 Omnibus Equity Plan.
Mr. DiPietro’s Arrangements
On May 26, 2017, Mr. DiPietro ceased to be our Executive Vice President, Human Resources and effective September 30, 2017, Mr. DiPietro ceased to be employed by us. We paid him the severance benefits payable under our executive severance policy for Executive Vice Presidents and certain additional benefits, as described in the CD&A above under the heading “2017 and 2018 Hiring- and Transition-Related Compensation Decisions—Mr. DiPietro’s Arrangements.”
Excise Tax Provisions
Before June 2009 we maintained an excise taxgross-up policy for all of our executives, including certain of our NEOs. Under this policy, if payments to these executive officers in the event of a corporate transaction or corporate change in control were subject to the excise tax under Internal Revenue Code Section 4999, we would pay the executive officer an additional amount that equals the amount of the excise tax, plus the income and other payroll taxes arising from our payment of the excise tax amount (280G taxgross-up), so that the executive officer realized the full intended benefit.
In June 2009 we changed our excise taxgross-up policy so that newly-hired executives are not eligible for any 280G taxgross-up but may elect to have severance payments reduced to an amount that will not be subject to excise tax. Consistent with this policy, as Ms. Alexander was already eligible for this benefit prior to June 2009, she remains eligible for a 280G tax gross up. No other current NEO is eligible for this benefit.
| | | | |
60 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued) |
Awards Under Equity Plans
Under the provisions of our equity plans, if a corporate change in control occurs, all outstanding options and stock awards under our equity plans granted prior to February 12, 2014, will become fully exercisable or vested, as the case may be, and options will remain exercisable until the original option expiration date.
As amended in February 2014, awards granted under our 2008 Omnibus Equity Plan on or after February 12, 2014, and awards granted our 2017 Omnibus Equity Plan, unless otherwise determined by our CompensationC&MD Committee at the time of grant, will not automatically vest or become exercisable upon a corporate change in control (i.e., upon a “single trigger”), butawards will vest or become exercisable in full immediately prior to an involuntary employment action that occurs within two years following a corporate change in control (i.e., upon a “double trigger”).
In the event of a corporate transaction, we can either cause the surviving corporation to assume all equity awards or accelerate their vesting and exercisability immediately
before the corporate transaction. If the equity awards are assumed and an executive officer’s employment is terminated in an involuntary employment action within two years following the corporate transaction, the equity awards that are assumed will become fully vested and, if applicable, exercisable. Under our equity plans, any assumed awards that become vested will remain exercisable through the earlier of 12 months from the termination date or the original option expiration date.
If the holder of an equity award retires, which is defined under our equity plans as leaving the employment of Biogen after reaching age 55 with 10 consecutive years of service, each then outstanding time-based equity award or earned performance-based equity award not yet vested or exercisable will become immediately vested or exercisable upon such termination at a rate of 50% of the shares unvested at the time of retirement plus an additional 10% of the shares for each full year of service beyond 10 years of service.service (and performance-based awards would remain eligible to vest based on actual performance). Options vested under these provisions remain exercisable for 36 months from retirement or until the original option expiration date, if sooner. Upon a termination of employment due to death or disability, all unvested time-based equity awards and earned performance-based equity awards vest in full and all unearned performance-based equity awards remain eligible to vest based on actual performance.
| | | | |
6159 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
Potential Post-Termination Payments Table
The following table summarizes the potential payments to each NEO under various termination events. The table assumes that the event occurred on December 31, 2017,2018, for all NEOs except for Dr. Scangos and Messrs. Clancy and DiPietro who separated from the Company during 2017.NEOs. The calculations use the closing price of our common stock as reported by Nasdaq on December 29, 2017, the last trading day of 2017,31, 2018, which was $318.57$300.92 per share.
| | | | | | | | | | | | |
Name and Payment Elements(1) (a) | | Retirement(2) (b) | | | Qualifying
Termination of
Employment Not Following a Corporate Transaction or Change in Control (c)(3) | | | Qualifying
Termination of
Employment
Following a Corporate Transaction or Change in Control (d)(3) | |
Michel Vounatsos(4) | | | | | | | | | | | | |
Severance | | | — | | | $ | 5,087,500 | | | $ | 6,325,000 | |
Performance-based RSUs | | | — | | | | — | | | $ | 14,407,305 | |
Medical, Dental and Vision | | | — | | | $ | 30,679 | | | $ | 40,905 | |
Outplacement(5) | | | — | | | $ | 32,000 | | | $ | 32,000 | |
Total | | | — | | | $ | 5,150,179 | | | $ | 20,805,210 | |
Jeffrey D. Capello | | | | | | | | | | | | |
Severance | | | — | | | $ | 1,275,000 | | | $ | 2,550,000 | |
Medical, Dental and Vision | | | — | | | $ | 19,954 | | | $ | 39,908 | |
Outplacement(5) | | | — | | | $ | 32,000 | | | $ | 32,000 | |
Total | | | — | | | $ | 1,326,954 | | | $ | 2,621,908 | |
Michael D. Ehlers(6) | | | | | | | | | | | | |
Severance | | | — | | | $ | 2,700,875 | | | $ | 2,700,875 | |
Performance-based RSUs | | | — | | | $ | 5,878,440 | | | $ | 5,878,440 | |
Time-based RSUs | | | — | | | $ | 659,440 | | | $ | 659,440 | |
Medical, Dental and Vision | | | — | | | $ | 42,088 | | | $ | 42,088 | |
Outplacement(5) | | | — | | | $ | 32,000 | | | $ | 32,000 | |
Total | | | — | | | $ | 9,312,843 | | | $ | 9,312,843 | |
Susan H. Alexander | | | | | | | | | | | | |
Severance | | | — | | | $ | 2,153,430 | | | $ | 2,461,063 | |
Performance-based RSUs | | | $3,881,140 | | | $ | 3,881,140 | | | $ | 6,468,566 | |
Medical, Dental and Vision | | | — | | | $ | 25,644 | | | $ | 29,308 | |
Outplacement(5) | | | — | | | $ | 32,000 | | | $ | 32,000 | |
280G TaxGross-Up(7) | | | — | | | | — | | | | — | |
Total | | | $3,881,140 | | | $ | 6,092,214 | | | $ | 8,990,937 | |
Paul F. McKenzie | | | | | | | | | | | | |
Severance | | | — | | | $ | 1,197,438 | | | $ | 2,052,750 | |
Performance-based RSUs | | | — | | | | — | | | $ | 3,940,587 | |
Time-based RSUs | | | — | | | | — | | | $ | 869,696 | |
Medical, Dental and Vision | | | — | | | $ | 23,547 | | | $ | 40,367 | |
Outplacement(5) | | | — | | | $ | 32,000 | | | $ | 32,000 | |
Total | | | — | | | $ | 1,252,985 | | | $ | 6,935,400 | |
Gregory F. Covino | | | | | | | | | | | | |
Severance | | | — | | | $ | 478,386 | | | $ | 521,875 | |
Performance-based RSUs | | | — | | | | — | | | $ | 672,178 | |
Time-based RSUs | | | — | | | | — | | | $ | 254,537 | |
Medical, Dental and Vision | | | — | | | $ | 19,023 | | | $ | 20,753 | |
Outplacement(5) | | | — | | | $ | 7,000 | | | $ | 7,000 | |
Total | | | — | | | $ | 504,409 | | | $ | 1,476,343 | |
George A. Scangos(8) | | | | | | | | | | | | |
Severance | | | — | | | $ | 7,244,877 | | | | — | |
Performance-based RSUs | | | — | | | $ | 14,780,047 | | | | — | |
Medical, Dental and Vision | | | — | | | $ | 7,227 | | | | — | |
Outplacement(5) | | | — | | | $ | 28,000 | | | | — | |
Total | | | — | | | $ | 22,060,151 | | | | — | |
Paul J. Clancy(9) | | | | | | | | | | | | |
Performance-based RSUs | | | $7,322,590 | | | | — | | | | — | |
Total | | | $7,322,590 | | | | — | | | | — | |
Kenneth A. DiPietro(10) | | | | | | | | | | | | |
Severance | | | — | | | $ | 2,019,413 | | | | — | |
Performance-based RSUs | | | — | | | $ | 903,189 | | | | — | |
Medical, Dental and Vision | | | — | | | $ | 23,750 | | | | — | |
Outplacement(5) | | | — | | | $ | 32,000 | | | | — | |
Total | | | — | | | $ | 2,978,352 | | | | — | |
| | | | |
62 | |  | |  |
| | |
5 | | Executive Compensation Matters (continued) |
| | | | | | | | | | | | | | | |
| | | |
Name and Payment Elements(1) (a) | | Retirement(2) (b) | | Qualifying Termination of Employment Not Following a Corporate Transaction or Change in Control (c)(3) | | Qualifying Termination of Employment Following a Corporate Transaction or Change in Control (d)(3) |
Michel Vounatsos(4) | | | | | | | | | | | | | | | |
Severance | | | | — | | | | $ | 4,680,000 | | | | $ | 6,240,000 | |
Performance-based RSUs | | | | — | | | | | — | | | | $ | 20,523,378 | |
Medical, Dental and Vision | | | | — | | | | $ | 29,709 | | | | $ | 39,613 | |
Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | |
Total | | | | — | | | | $ | 4,741,709 | | | | $ | 26,834,991 | |
Jeffrey D. Capello | | | | | | | | | | | | | | | |
Severance | | | | — | | | | $ | 1,487,500 | | | | $ | 2,550,000 | |
Performance-based RSUs | | | | | | | | | — | | | | $ | 2,736,395 | |
Medical, Dental and Vision | | | | — | | | | $ | 22,611 | | | | $ | 38,761 | |
Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | |
Total | | | | — | | | | $ | 1,542,111 | | | | $ | 5,357,156 | |
Michael D. Ehlers | | | | | | | | | | | | | | | |
Severance | | | | — | | | | $ | 1,890,613 | | | | $ | 2,835,920 | |
Performance-based RSUs | | | | — | | | | | — | | | | $ | 7,240,328 | |
Time-based RSUs | | | | — | | | | | — | | | | $ | 1,736,609 | |
Medical, Dental and Vision | | | | — | | | | $ | 25,574 | | | | $ | 38,361 | |
Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | |
Total | | | | — | | | | $ | 1,948,187 | | | | $ | 11,883,218 | |
Susan H. Alexander | | | | | | | | | | | | | | | |
Severance | | | | — | | | | $ | 2,228,802 | | | | $ | 2,547,202 | |
Performance-based RSUs | | | $ | 4,671,689 | | | | $ | 4,671,689 | | | | $ | 6,673,841 | |
Time-based RSUs | | | $ | 852,055 | | | | $ | 852,055 | | | | $ | 1,217,221 | |
Medical, Dental and Vision | | | | — | | | | $ | 23,544 | | | | $ | 26,908 | |
Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | |
280G TaxGross-Up(6) | | | | — | | | | | — | | | | | — | |
Total | | | $ | 5,523,744 | | | | $ | 7,808,090 | | | | $ | 10,497,172 | |
Paul F. McKenzie | | | | | | | | | | | | | | | |
Severance | | | | — | | | | $ | 1,436,926 | | | | $ | 2,155,389 | |
Performance-based RSUs | | | | — | | | | | — | | | | $ | 5,463,914 | |
Time-based RSUs | | | | — | | | | | — | | | | $ | 1,688,161 | |
Medical, Dental and Vision | | | | — | | | | $ | 26,183 | | | | $ | 39,274 | |
Outplacement(5) | | | | — | | | | $ | 32,000 | | | | $ | 32,000 | |
Total | | | | — | | | | $ | 1,495,109 | | | | $ | 9,378,738 | |
Notes to the Potential Post-Termination Payments Table
| (1) | In the event of an executive’s death or disability, all outstanding time-based equity awards and earned performance-based equity awards under the Company’s LTI program will vest in full.full and all unearned performance-based equity awards will remain outstanding and eligible to vest based on actual performance. The value of such accelerated awards for all NEOs would be the same amount as shown in column (d) for such NEO (based on actual performance estimated as of December 31, 2017)2018). |
| (2) | Ms. Alexander and Mr. Clancy werewas eligible for potential payments upon retirement at December 31, 2017.2018. Upon retirement, 70% of any vested CSPU awards would be paid following, if applicable, thesix-month delay required by Section 409A of the Internal Revenue Code, 70% of any unvested CSPU awards would vest immediately upon certification of the achievement of the applicable performance criteriagoals and would be paid following, if applicable, thesix-month delay required by Section 409A of the Internal Revenue CodeCode. Any unvested PSU and any unvested MSU awards would, subject to the achievement of any applicable performance criteria,goals, remain outstanding and vest in accordance with the terms of such awards.awards based on actual performance as to 70% of the earned shares. The amount listed in column (b) assumesis the estimated value of 70% of all unvested awards held by Ms. Alexander, based on actual performance estimated as of December 31, 2017.2018, for unearned performance-based awards. Based on years of service, Ms. Alexander and Mr. Clancy werewas eligible for continuedaccelerated vesting on 60% and 100%70% of her outstanding awards respectively.as of December 31, 2018. |
| (3) | The amounts listed in column (c) and column (d) for Performance-based RSUs for the applicable named executive officers assumesincludes the value of applicable unvested awards based on actual performance estimated as of December 31, 2017.2018. |
| (4) | Pursuant to his employment agreement, effective January 6, 2017, upon an involuntary termination by the Company without cause or involuntary employment action not following a corporate transaction or change in control,CIC, Mr. Vounatsos is eligible to receive a lump sum payment within 60 days |
| | | | |
| 60 | |  | |  |
| | |
| 5 | | Executive Compensation Matters (continued) |
| consisting of the pro rata portion of the target bonus for the year of termination and an amount equal to the sum of the annual base salary annual rate and target bonus in effect at the time of termination multiplied by a factor of 1.5, continuation of medical, dental and vision insurance for up to 18 months and up to 12 months of executive outplacement services. Upon an involuntary termination by the Company without cause or an involuntary employment action following a corporate transaction or change in control,CIC, Mr. Vounatsos is eligible to receive a lump sum payment within 60 days consisting of the pro rata portion of the target bonus for the year of termination and an amount equal to the sum of the annual base salary rate and target bonus in effect at the time of termination multiplied by a factor of 2.0, continuation of medical, dental and vision insurance for up to 24 months and up to 12 months of outplacement services. |
| (5) | The named executive officers are provided outplacement services at a cost of up to $32,000 for the Executive Vice President level and $7,000 for the Vice President level except for Dr. Scangos, who was eligible for outplacement services at a cost of $28,000 based the cost of the program offered to executives on his termination date of January 6, 2017; however, Dr. Scangos did not utilize this benefit.level. |
| (6) | Dr. Ehlers would be entitled to the payments in column (d) in connection with a termination as discussed above under the heading “Potential Payments Upon Termination or Change in Control—Executive Severance Policy—Dr. Ehlers’ Additional Arrangements.” |
(7) | The payments for Ms. Alexander upon a corporate transaction or a corporate change in control on December 31, 2017,2018, would not have been subject to a Section 280G excise tax. |
(8) | On January 6, 2017, Dr. Scangos ceased to be our Chief Executive Officer and became entitled to the payments in column (c), as further described in the CD&A under the heading “2017 and 2018 Hiring- and Transition-Related Compensation Decisions — Dr. Scangos’ Arrangements.” As part of his employment agreement, Dr. Scangos was also eligible to receive a severance payment equal to the pro rata portion of the 2017 bonus, subject to performance determination. The amount of the prorated annual bonus equaled $44,877. In addition, Dr. Scangos was eligible to receive nine months of executive-level outplacement services at our cost equaling $28,000; however, Dr. Scangos did not utilize this benefit. |
(9) | Mr. Clancy voluntarily separated from the Company on July 1, 2017. Mr. Clancy’s outstanding LTI awards were eligible for retirement vesting and continue to vest in accordance with the service requirements of the original awards. |
(10) | On September 30, 2017, Mr. DiPietro’s employment terminated, at which time he became entitled to the payments in column (c), as further described in the CD&A under the heading “2017 and 2018 Hiring- and Transition-Related Compensation Decisions — Mr. DiPietro’s Arrangements.” In addition, Mr. DiPietro was eligible to receive 12 months of executive-level outplacement services at our cost equaling $32,000; however, Mr. DiPietro did not utilize this benefit. |
CEO Pay Ratio
We believe executive pay must be internally consistent and equitable to motivate our employees to create stockholder value and we are committed to internal pay equity. As discussed earlier in this Proxy Statement, our compensation programs are designed to drive the creation of long-term stockholder value by delivering performance-based compensation. We invest in our employees at all levels in the Company by rewarding performance that balances risk and reward, empowering professional growth and development and by offering affordable benefits and programs that meet the diverse needs of our employees.
We believe strongly inpay-for-performance and all of our employees are eligible to participate in our annual bonus plan, our LTI programs and our benefit plans. Our annual bonus plan is consistently maintained for all participants globally, with the same Company performance goals, payout levels (as a percentage of target) and administrative provisions regardless of the participant’s job level, location or function in the Company. We also have a long-term incentive program that provides different forms of awards depending upon an employee’s level but is otherwise consistent throughout the Company.
The following is a reasonable estimate, prepared under applicable SEC rules, of the ratio of the annual total compensation of our CEO to the median of the annual total compensation of our other employees. We determinedUnder SEC rules, we used the same median employee as we used for our 2017 pay ratio because we reasonably believe there have been no changes to our employee population or compensation programs that would result in a significant change to our pay ratio disclosure. The methodology used to identify our median employee asfor our 2017 pay ratio is described in our 2018 proxy statement.
Our median employee is a full-time employee based in the U.S. In October 2017, when we determined the median employee, approximately 59% of October 6, 2017,our workforce was based on a consistently applied compensation measure defined asin the sumU.S. with the remaining approximately 41% of base salary, target bonus and LTI target value. our workforce based in the rest of the world. In addition, approximately 98% of our workforce was full-time.
For the identifiedour median employee, annual total compensation was then calculated in accordance with the SEC’s rules for the Summary Compensation Table.Table, including salary, bonus, LTI grant date fair value and value of certain benefits provided, including relocation benefits. For our CEO, annual total compensation was equivalentis equal to the amount included in the “Total” column of the Summary Compensation Table, value with the exception of base salary, whichand our CEO’s annual total compensation for 2018 was annualized to $1,100,000 based on the rate approved coincident with Mr. Vounatsos’ appointment to the CEO role effective January 6, 2017.$16,168,646. The annual total compensation of the median employee (excluding our CEO) for 20172018 was $148,904$170,521, including base salary, annual bonus, LTI grant value, and our CEO’s annual total compensation for 2017 was $13,676,488.certain benefits includingone-time relocation benefit. Based on the foregoing, our estimate of the ratio of the annual total compensation of our CEO to the median of the annual total compensation of our other employees was 9295 to 1. This pay ratio is a reasonable estimate calculated in a manner consistent with SEC rules based on our payroll and employment records and the methodology described above. Given the different methodologies, estimates, assumptions and exclusions that variousother public companies will use to determine an estimate of their pay ratio, the estimated ratio reported above should not be used as a basis for comparison between companies.
| | | | |
6361 | |  | |  |
| | | | |
| | | | |
| | Proposal 4 – Stockholder Proposal Requesting Certain Proxy Access Bylaw AmendmentsAdditional Information
| | |
| | | | |
Mr. James McRitchie and Ms. Myra K. Young (whom we sometimes refer to as the Proposal 4 Proponents) have notified us that they intend to submit the following proposal for consideration at the Annual Meeting. The Proposal 4 Proponents have indicated that they beneficially own 50 shares of our common stock. We will provide their address promptly upon a stockholder’s oral or written request. The
Proposal 4 Proponents are responsible for the content of the proposal, for which we and our Board of Directors accept no responsibility. The proposal will be voted on at the Annual Meeting if the Proposal 4 Proponents, or a qualified representative, is present at the Annual Meeting and submits the proposal for a vote. Our statement in opposition follows the proposal.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTEAGAINST THE APPROVAL
OF THIS STOCKHOLDER PROPOSAL.
Proposal 4 – Stockholder Proxy Access Amendments
RESOLVED: Stockholders of Biogen Inc. (the “Company”) ask the board of directors (the “Board”) to amend its proxy access bylaw provisions and any associated documents, to include the following changes for the purpose of (1) decreasing the average amount of Company common stock the average member of a nominating group would be required to hold for three years to satisfy the aggregate ownership requirements to form a nominating group and (2) decreasing the barriers forre-nomination:
1. | No limitation shall be placed on the number of stockholders that can aggregate their shares to achieve the 3% of common stock required to nominate directors under our Company’s proxy access provisions. |
2. | No limitation shall be placed on there-nomination of stockholder nominees based on the number or percentage of votes received in any election. |
Supporting Statement: Under current provisions, even if the 20 largest public pension funds were able to aggregate their shares, they would not meet the 3% holding criteria at most of companies examined by the Council of Institutional Investors. Allowing an unlimited number of shareholders to aggregate shares would facilitate greater participation by individuals and institutional investors in meeting the stock ownership requirements, 3% of the outstanding common stock entitled to vote.
The SEC’s universal proxy access Rule14a-11(https://www.sec.gov/rules/final/2010/33-9136.pdf) was vacated after a court decision regarding the SEC’s cost-benefit analysis. Therefore, proxy access rights must be established on acompany-by-company basis. Subsequently, a cost-benefit analysis by CFA Institute,ProxyAccess in the
United States: Revisiting the Proposed SEC Rule (http://www.cfapubs.org/doi/pdf/10.2469/ccb.v2014.n9.1), found proxy access would “benefit both the markets and corporate boardrooms, with little cost or disruption,” raising US market capitalization by up to $140.3 billion.
Proxy Access: Best Practices 2017
(http://www.cii.org/files/publications/misc/Proxy_Access_2017_FINAL.pdf) by the Council of Institutional Investors (CII), notes “while proxy access has gained broad acceptance, some adopting companies have included, or are considering including, provisions that could significantly impair shareholders’ ability to use it.”
Governance Changes through Shareholder Initiatives(https://cba.unl.edu/academic-programs/departments/finance/about/seminar-series/documetnts/llieve.pdf) found the announcement of shareholder proposals for proxy access submitted to 75 U.S. public companies resulted in $10.6 billion of increased shareholder value across targeted firms.
Although the Company’s Board adopted a proxy access bylaw, it contains troublesome provisions that significantly impair the ability of shareholders to participate because of the large average amount of common shares each is required to hold for three years given the current aggregation limit of 20 and the ability of shareholder nominees to run again. Adoption of the requested amendments would come closer to meeting best practices as described by CII. Last year dozens of funds voted FOR a similar proposal, including Wells Fargo Advisors, Invesco Advisors and PNC Capital Advisors. The proposal is especially important shareholders, given Company underperformance relative to the Nasdaq.
| | | | |
64 | |  | |  |
| | |
6 | | Stockholder Proposals(continued) |
Increase Stockholder Value
Vote for Stockholder Proxy Access Amendments – Proposal 4
Company Statement in Opposition
Our Board of Directors recommends that stockholders vote AGAINST this stockholder proposal for the following reasons:
Our proxy access framework, the result of discussions with the Proposal 4 Proponents in 2015, strikes the right balance between promoting stockholder nomination rights and protecting the interests of all our stockholders.
In 2015 we adopted a proxy access bylaw that is within the mainstream of other significant U.S. public companies with proxy access rights and that is more favorable by allowing a stockholder or group to nominate up to 25% of the Board (but not fewer than one nominee) as opposed to the more standard 20% of the Board.
Specifically, our Bylaws permit a stockholder, or a group of up to 20 stockholders, owning at least 3% of our outstanding shares of common stock continuously for at least 3 years, to nominate and include in our annual meeting proxy materials director nominees constituting 25% of the Board (but no fewer than one nominee), subject to the other procedural requirements specified in our Bylaws, which is posted on our website,www.biogen.com, under the “Corporate Governance” subsection of the “Investors” section of our website.
Our Board adopted a regime that it believed struck the appropriate balance between providing a workable process that can be used if ever needed and that reinforces our Board’s accountability, while mitigating the possibility of proxy access being used by stockholders pursuing objectives that are not broadly supported by other stockholders or otherwise misusing proxy access.
The Proposal 4 Proponents submitted a proxy access stockholder proposal in 2015. After we adopted proxy access, they withdrew their proposal. As described below, the changes advocated by the stockholder proposal are not necessary for the proper functioning of proxy access.
We have provided an effective and workable proxy access framework. The requested changes are unnecessary and potentially disruptive.
Our proxy access bylaw permits groups of up to 20 stockholders to aggregate their shares to reach the required 3% ownership threshold (with a group of funds under common
management and investment control counting as a single stockholder). The Proposal 4 Proponents’ requested change to our proxy access bylaw would place no limit on the number of stockholders who may aggregate their holdings to reach the required 3% ownership threshold. We believe that permitting a group of unlimited size to use the proxy access bylaw provisions could be unworkable and could impose a significant cost and administrative burden on the Company to verify that the eligibility and procedural requirements have been satisfied (and continue to be satisfied through the annual meeting date) by each of the potentially very large number of stockholders in the aggregate pool.
We believe an aggregation limit of 20 provides abundant opportunities for the Company’s stockholders to combine with other stockholders to satisfy the ownership requirement, provided that they also satisfy the required holding period requirement. In addition, a20-stockholder limit is widely embraced by companies adopting proxy access, and widely endorsed among institutional stockholders’ voting policies.
Our proxy access bylaw provides that a candidate who does not receive at least 25% of the votes cast in favor of such candidate’s election will not be nominated as a proxy access candidate for the following two years. This is designed solely to prevent stockholders from abusing the proxy access process, subjecting the Company and other stockholders to the expense and effort of responding to proxy access, after that process has already been used once with a particular candidate whom stockholders as a whole did not meaningfully support.
We have a strong corporate governance structure and record of accountability.
The Company’s corporate governance structure reflects our commitment to strong and effective governance practices and a willingness to be responsive and accountable to stockholders. We regularly assess our corporate governance policies to take into account evolving best practices and to address stockholder feedback. Our goals are to align the interests of stockholders, directors and management; ensure accountability; encourage robust engagement with our key stakeholders and provide our stockholders with a meaningful voice in both the nomination and the election of directors. Some of our governance policies and practices that support these goals are:
Annual elections of all directors.
Majority voting in uncontested director elections and resignation policy.
| | | | |
65 | |  | |  |
| | |
6 | | Stockholder Proposals(continued) |
Our Board comprises a substantial majority of independent directors and all standing committees of our Board comprise only independent directors.
We have an independent Chairman with significant responsibilities, including:
presiding at meetings of our Board and executive sessions of our independent directors;
reviewing and assisting in setting the agenda and schedule for our Board meetings in collaboration with our Chief Executive Officer; and
serving as a liaison for stockholder communications with our Board.
Stockholders representing 25% or more of our outstanding shares can call a special meeting of stockholders.
Our stockholders are able to:
recommend director candidates to our Corporate Governance Committee, which considers such recommendations in the same manner as recommendations received from other sources;
directly nominate director candidates and solicit proxies for the election of those candidates in accordance with our Bylaws and the federal securities laws; and
communicate directly with members of our Board, the Chairman, any Board committee or our independent directors as a group.
The robust proxy access provisions our Board has adopted, together with these other practices, promote Board independence and provide substantial opportunities consistent with best practices for stockholder input into the governance process. The changes to proxy access requested by the proposal are unnecessary and disrupt the balanced approach reflected in our Bylaws.
| | | | |
| | | | |
| | Proposal 5 – Stockholder Proposal Requesting a Report on the Extent to Which Risks Related to Public Concern Over Drug Pricing Strategies are Integrated into Incentive Compensation Arrangements
| | |
| | | | |
Azzad Asset Management(co-sponsored with Boston Common Asset Management, LLC; Domini Impact Equity Fund; Mercy Investment Services, Inc.; Missionary Oblates of Mary Immaculate OIP Investment Trust; Northwest Women Religious Investment Trust; Sisters of St. Francis Charitable Trust; Trinity Health; and UAW Retiree Medical Benefits Trust) (we sometimes refer to these entities as the Proposal 5 Proponents) have notified us that it intends to submit the following proposal for consideration at the Annual Meeting. Azzad Asset Management has indicated
that they beneficially own 6.607 shares of our common stock. We will provide the Proposal 5 Proponents’ address promptly upon a stockholder’s oral or written request. The Proposal 5 Proponents are responsible for the content of the proposal, for which we and our Board accept no responsibility. The proposal will be voted on at the Annual Meeting if the Proposal 5 Proponents, or a qualified representative, is present at the Annual Meeting and submits the proposal for a vote. Our statement in opposition follows the proposal.
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTEAGAINST THE APPROVAL
OF THIS STOCKHOLDER PROPOSAL.
RESOLVED, that shareholders of Biogen Inc. (“Biogen”) urge the Compensation Committee to report annually to shareholders on the extent to which risks related to public concern over drug pricing strategies are integrated into Biogen’s incentive compensation policies, plans and programs (together, “arrangements”) for senior executives. The report should include, but need not be limited to, discussion of whether incentive compensation arrangements reward, or
not penalize, senior executives for (i) adopting pricing strategies, or making and honoring commitments about pricing, that incorporate public concern regarding the level or rate of increase in prescription drug prices; and (ii) considering risks related to drug pricing when allocating capital.
| | | | |
66 | |  | |  |
| | |
6 | | Stockholder Proposals(continued) |
SUPPORTING STATEMENT
As long-term investors, we believe that senior executive incentive compensation arrangements should reward creation of sustainable long-term value. To that end, it is important that those arrangements align with company strategy and encourage responsible risk management.
A key risk facing pharmaceutical companies is backlash against high drug prices. Public outrage over high prices and their impact on patient access may force price rollbacks and harm corporate reputation. Legislative or regulatory investigations regarding pricing of prescription medicines may bring about broader changes. (E.g.,https://democrats-oversight.house.gov/news/press-releases/cummings-and-welch-launch-investigation-of-drug-companies-skyrocketing-prices;https://democrats-oversight.house.gov/news/press-releases/cummings-and-welch-propose-medicare-drug-negotiation-bill-in-meeting-with)
Biogen was publicly criticized in 2017 for the $750,000first-year price tag, and $375,000 annual cost thereafter, for new spinal muscular atrophy treatment Spinraza. (E.g., https://www.npr.org/sections/health-shots/2017/08/01/540100976/drug-puts-a-750-000-price-tag-on-life) Congressional attention has also recently focused on the price of drugs for multiple sclerosis, including those sold by Biogen.(https://www.investors.com/news/technology/biogen-teva-slip-after-democrats-launch-ms-drug-pricing-probe/)
We are encouraged by Biogen’s improved transparency on pricing. We are concerned, however, that the incentive compensation arrangements applicable to Biogen’s senior executives may not encourage senior executives to take actions that result in lower short-term financial performance even when those actions may be in Biogen’s best long-term financial interests.
Biogen uses revenue and earnings per share as metrics for the annual bonus (together with strategic goals), and revenue and free cash flow as the metrics for the cash settled performance units program. (2017 Proxy Statement, at38-41) A recent Credit Suisse analyst report found that “US drug price rises contributed 100% of industry EPS growth in 2016” and characterized that fact as “the most important issue for a Pharma investor today.” The report identified Biogen as a company where U.S. net price increases accounted for at least 100% of 2016 EPS growth. (GlobalPharma and Biotech Sector Review: Exploring Future US Pricing Pressure, Apr. 18, 2017, at 1)
In our view, excessive dependence on drug price increases is a risky and unsustainable strategy, especially when price hikes drive large senior executive payouts. For example, media coverage noted that a 600% rise in Mylan’s CEO’s total compensation accompanied the 400% EpiPen price increase. (See,e.g., https://www.nbcnews.com/business/consumer/mylan-execs-gave-themselves-raises-they-hiked-epipen-prices-n636591;https://www.wsj.com/articles/epipen-maker-dispenses-outsize-pay-1473786288;https://www.marketwatch.com/story/mylan-top-executive-pay-was-second-highest-in-industry-just-as-company-raised-epipen-prices-2016-09-13)
The requested disclosure would allow shareholders to assess the extent to which compensation arrangements encourage senior executives to responsibly manage risks relating to drug pricing and contribute to long-term value creation. We urge shareholders to vote for this Proposal.
Company Statement in Opposition
Our Board of Directors recommends that stockholders vote AGAINST this stockholder proposal for the following reasons:
Our annual proxy statement provides detailed information on the material factors upon which our incentive compensation for executive officers is based and therefore covers the information that would be included in the report called for by this stockholder proposal.
Each year, the CD&A section of our proxy statement describes our executive compensation philosophy, our Compensation Committee’s process for setting executive compensation, the elements of our compensation program, the factors our Compensation Committee considered when setting executive compensation for the previous year and the factors that affected incentive payouts for the previous year’s performance.
Our proxy statements include detailed analyses of our Compensation Committee’s review of the compensation paid to individual named executive officers. These analyses provide all relevant information about the material factors that were considered in those executives’ compensation. These analyses include information regarding specific individualized performance goals, including goals relating to strategic efforts and decisions. This is information that is broadly available to stockholders and the general public.
Further, as part of its review of the CD&A, our Compensation Committee is required to assess the risk associated with our compensation policies and decisions. In connection
| | | | |
67 | |  | |  |
| | |
6 | | Stockholder Proposals(continued) |
with this assessment, we provide robust disclosure in the CD&A about the factors, including risk, that our Compensation Committee considers when determining executive compensation. Our CD&A thus makes it clear that the potential risks of our incentive compensation programs — including long-term risks, which are a central focus of this stockholder proposal — are already assessed and weighed by our Compensation Committee when it approves executive compensation.
In addition, our Compensation Committee’s charter sets forth the duties and responsibilities of our Compensation Committee, which include, among other things, determining and approving the compensation of our CEO and other executive officers. The charter makes clear that the duties and responsibilities of our Compensation Committee encompass a review of significant risks with respect to our
compensation and benefits program and steps taken by management to monitor and mitigate such risk. Furthermore, our compensation philosophy is performance based. Each year we establish performance targets that are based on financial, market share, pipeline development and other goals that we deem relevant for creation of stockholder value. Underlying these targets are consideration of, among other things, overall market growth, competitive threats, new product introductions, unit growth and product pricing.
The report the Proposal 5 Proponents ask for would contain no material information that is not already evident from the information that we are required to, and do, disclose in our CD&A. Accordingly, we believe an annual report is unnecessary.
| | | | |
68 | |  | |  |
STOCK OWNERSHIP
The following table and accompanying notes provide information about the beneficial ownership of our common stock by:
each stockholder known by us to be the beneficial owner of more than 5% of our common stock;
each of our named executive officers;
each of our directors and nominees for director; and
all of our directors and executive officers as a group.
Except as otherwise noted, the persons identified have sole voting and investment power with respect to the shares of our common stock beneficially owned. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting and investment power with respect to the shares. Except as otherwise noted, the information below is as of April 12, 201819, 2019 (Ownership Date).
Unless otherwise indicated in the footnotes, the address of each of the individuals named below is: c/o Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142.
| | Name | | Shares
Owned (1) | | Shares Subject to
Options and
Stock Units (2) | | Total Number of
Shares Beneficially
Owned | | Percentage of
Outstanding
Shares (3) | | | Shares
Owned(1) | | Shares Subject to Options and Stock Units(2) | | Total Number of Shares Beneficially Owned(1) | | Percentage of Outstanding Shares(3) | |
5% Stockholders | | | | | | | | | | | | | | | | |
BlackRock Inc.(4) 55 East 52nd Street New York, NY 10055 | | | 16,804,682 | | | | — | | | | 16,804,682 | | | | 7.96 | % | | | 16,568,963 | | | | — | | | | 16,568,963 | | | | 8.2 | % |
PRIMECAP Management Company(5) 177 East Colorado Boulevard 11th Floor Pasadena, CA 91105 | | | 15,109,231 | | | | — | | | | 15,109,231 | | | | 7.16 | % | |
The Vanguard Group(6) 100 Vanguard Boulevard Malvern, PA 19355 | | | 14,871,019 | | | | — | | | | 14,871,019 | | | | 7.05 | % | |
The Vanguard Group(5) 100 Vanguard Boulevard Malvern, PA 19355 | | | | 15,170,607 | | | | — | | | | 15,170,607 | | | | 7.52 | % |
PRIMECAP Management Company(6) 177 East Colorado Boulevard 11th Floor Pasadena, CA 91105 | | | | 14,727,267 | | | | — | | | | 14,727,267 | | | | 7.31 | % |
Named Executive Officers | | | | | | | | | | | | | | | | |
Michel Vounatsos(7) | | | 8,432 | | | | 3,244 | | | | 11,676 | | | | * | | | | 16,657 | | | | 3,252 | | | | 19,909 | | | | * | |
Jeffrey D. Capello | | | — | | | | — | | | | — | | | | — | | | | 917 | | | | — | | | | 917 | | | | * | |
Michael Ehlers(7) | | | 2,904 | | | | 3,528 | | | | 6,432 | | | | * | | | | 6,824 | | | | 3,538 | | | | 10,362 | | | | * | |
Susan H. Alexander | | | 24,844 | | | | 6,395 | | | | 31,239 | | | | * | | | | 31,976 | | | | — | | | | 31,976 | | | | * | |
Paul F. McKenzie | | | 2,557 | | | | — | | | | 2,557 | | | | * | | | | 6,407 | | | | — | | | | 6,407 | | | | * | |
Gregory F. Covino | | | 4,021 | | | | — | | | | 4,021 | | | | * | | |
George A. Scangos(8) | | | 89,751 | | | | — | | | | 89,751 | | | | * | | |
Paul J. Clancy(9) | | | 13,525 | | | | — | | | | 13,525 | | | | * | | |
Kenneth A. DiPietro(10) | | | 913 | | | | — | | | | 913 | | | | * | | |
Directors | | | | | | | | | | | | | | | | |
Alexander J. Denner(11) | | | 422,832 | | | | 1,055 | | | | 423,887 | | | | * | | |
John R Chiminski | | | | — | | | | — | | | | — | | | | — | |
Alexander J. Denner(8) | | | | 534,687 | | | | 880 | | | | 535,567 | | | | * | |
Caroline D. Dorsa | | | 17,117 | | | | 1,055 | | | | 18,172 | | | | * | | | | 18,172 | | | | 880 | | | | 19,052 | | | | * | |
William A. Hawkins | | | | — | | | | — | | | | — | | | | — | |
Nancy L. Leaming | | | 9,008 | | | | 1,055 | | | | 10,063 | | | | * | | | | 10,063 | | | | 880 | | | | 10,943 | | | | * | |
Jesus B. Mantas | | | | — | | | | — | | | | — | | | | — | |
Richard C. Mulligan | | | 8,974 | | | | 1,055 | | | | 10,029 | | | | * | | | | 10,029 | | | | 880 | | | | 10,909 | | | | * | |
Robert W. Pangia | | | 16,652 | | | | 7,169 | | | | 23,821 | | | | * | | | | 17,707 | | | | 880 | | | | 18,587 | | | | * | |
Stelios Papadopoulos(12) | | | 28,206 | | | | 1,740 | | | | 29,946 | | | | * | | |
Stelios Papadopoulos(9) | | | | 29,946 | | | | 1,450 | | | | 31,396 | | | | * | |
Brian S. Posner | | | 5,275 | | | | 1,055 | | | | 6,330 | | | | * | | | | 6,015 | | | | 880 | | | | 6,895 | | | | * | |
Eric K. Rowinsky | | | 13,089 | | | | 1,055 | | | | 14,144 | | | | * | | | | 14,144 | | | | 880 | | | | 15,024 | | | | * | |
Lynn Schenk(13) | | | 9,074 | | | | 1,055 | | | | 10,129 | | | | * | | |
Lynn Schenk(10) | | | | 10,097 | | | | 880 | | | | 10,977 | | | | * | |
Stephen A. Sherwin | | | 4,079 | | | | 13,333 | | | | 17,412 | | | | * | | | | 4,284 | | | | 13,158 | | | | 17,442 | | | | * | |
Executive officers and directors as a group (19 persons)(7)(14) | | | 581,710 | | | | 42,794 | | | | 624,504 | | | | * | | |
Executive officers and directors as a group (23 persons)(7)(11) | | | | 731,239 | | | | 28,438 | | | | 759,677 | | | | * | |
| * | Represents beneficial ownership of less than 1% of our outstanding shares of common stock. |
| | | | |
6962 | |  | |  |
| | |
76 | | Additional Information (continued) |
| (1) | The shares described as “owned” are shares of our common stock directly or indirectly owned by each listed person, rounded up to the nearest whole share. |
| (2) | Includes options that are or will become exercisable and RSUs and MSUs that will vest within 60 days of the Ownership Date. |
| (3) | The calculation of percentages is based upon 211,007,835193,893,397 shares outstanding on the Ownership Date, plus for each of the individuals listed above the shares subject to options and RSUs and MSUs exercisable within 60 days of the Ownership Date, as reflected in the column under the heading “Shares Subject to Options and Stock Units.” |
| (4) | Based solely on information as of December 31, 2017,2018, contained in a Schedule 13G/A filed with the SEC by BlackRock Inc. on January 29, 2018,February 4, 2019, which also indicates that it has sole voting power with respect to 14,879,33114,695,569 shares and sole dispositive power with respect to 16,804,68316,568,963 shares. |
| (5) | Based solely on information as of December 31, 2017,2018, contained in a Schedule 13G/A filed with the SEC by The Vanguard Group on February 11, 2019, which also indicates that it has sole voting power with respect to 246,897 shares, sole dispositive power with respect to 14,880,929 shares, shared voting power with respect to 47,063 shares and shared dispositive power with respect to 289,678 shares. |
| (6) | Based solely on information as of December 31, 2018, contained in a Schedule 13G/A filed with the SEC by PRIMECAP Management Company on February 27, 2018,8, 2019, which also indicates that it has sole voting power over 1,930,7301,696,975 shares and sole dispositive power over 15,109,23114,727,267 shares. |
(6) | Based solely on information as of December 31, 2017, contained in a Schedule 13G/A filed with the SEC by The Vanguard Group on February 8, 2018, which also indicates that it has sole voting power with respect to 302,180 shares, sole dispositive power with respect to 14,530,150 shares, shared voting power with respect to 46,671 shares and shared dispositive power with respect to 340,869 shares. |
| (7) | Includes shares underlying MSUs that will vest within 60 days of the Ownership Date, assuming the maximum possible number of shares that are eligible for vesting on the vesting date. The actual number of shares that will vest on each vesting date will be determined by comparing the price of Biogen common stock on such vesting date to the price on the grant date (i.e., number of vested shares = number of shares at target payout times the[30-day average closing stock price ending on the vesting date divided by the30-day average closing stock price on the grant date]). |
| (8) | Includes 10,756383,858 shares held in trusts of which Dr. Scangos isbeneficially owned by Sarissa Capital Offshore Master Fund LP, a trustee. Dr. Scangos ceased to be Biogen’s Chief Executive Officer and a member of our Board, effective January 6, 2017. Dr. Scangos’s stock ownership is based on Company information. |
(9) | Mr. Clancy voluntarily separated from the Company effective July 1, 2017. |
(10) | Mr. DiPietro ceased to be Biogen’s Executive Vice President, Human Resources effective May 26, 2017, and ceased to be employed by us effective September 30, 2017. |
(11) | Includes (i) 30,000Cayman Islands exempted limited partnership (Sarissa Offshore), 79,800 shares of common stock directly beneficially owned by Sarissa Capital Catapult Fund LLC, a Delaware limited liability company (Sarissa Catapult); and (ii) 383,85861,000 shares of common stock directly beneficially owned by Sarissa Capital Offshore Master Fund LP, a Cayman Islands limited partnership (Sarissa Offshore and, together with Sarissa Catapult, the Sarissa Funds). Sarissa Capital Management GP LLC, a Delaware limited liability company (Sarissa Capital GP), is the general partner of Sarissa Capital Management LP, a Delaware limited partnership (Sarissa Capital),. Sarissa Capital is the investment advisor to thecertain investment funds, including Sarissa Funds.Offshore and Sarissa Catapult. Dr. Denner is the Chief Investment Officer of Sarissa Capital and controls the ultimate general partner of each of Sarissa Capital and Sarissa Offshore and the managing member of Sarissa Capital GP.Catapult. By virtue of the foregoing, Dr. Denner may be deemed to indirectly beneficially own (as that term is defined in Rule13d-3 of the Exchange Act) the shares that the Sarissa Funds directlythose entities beneficially own. Dr. Denner disclaims beneficial ownership of suchthese shares except to the extent of common stock owned by the Sarissa Funds.any pecuniary interest therein. |
(12)(9) | Includes 28,206 shares held in limited liability companies of which Dr. Papadopoulos is the sole manager. |
(13)(10) | Includes 738 shares held in a trust of which Ms. Schenk is a trustee and 2,362 shares held in a defined benefit plan. |
(14)(11) | Includes 445,164555,964 shares held indirectly through trusts, funds, defined benefit plans or limited liability companies. |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our executive officers, directors and greater than 10% stockholders to file initial reports of ownership and changes of ownership of our common stock. As a practical matter, we assist our directors and executive officers by monitoring transactions and completing and filing Section 16 forms on their behalf. Based solely on information provided to us by our directors and executive officers, we believe that during 20172018 all such parties complied with all applicable filing requirements.
| | | | |
7063 | |  | |  |
| | |
76 | | Additional Information (continued) |
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
Our Code of Business Conduct (Values in Action), Corporate Governance Principles, Related Person Transaction Policy and Conflict of Interest Policy set forth our policies and procedures for the review and approval of transactions with related persons, including transactions that would be required to be disclosed in this Proxy Statement in accordance with SEC rules.
In circumstances where one of our directors or executive officers, or a family member, has a direct or indirect material interest in a transaction involving Biogen, our Corporate Governance Committee must review and approve all such proposed transactions or courses of dealing. In determining whether to approve or ratify a transaction with a related person, among the factors our Corporate Governance Committee may consider (as applicable) are:
the business reasons for entering into the transaction;
the size of the transaction and the nature of the related person’s interest in the transaction;
whether the transaction terms are as favorable to us as they would be to an unaffiliated third party;
whether the transaction terms are more favorable to the related person than they would be to an unaffiliated third party;
the availability of alternative sources for comparable products, services or other benefits;
whether the transaction would impair the independence or judgment of the related person in the performance of his or her duties to us;
fornon-employee directors, whether the transaction would be consistent with Nasdaq’s requirements for independent directors;
whether the transaction is consistent with our Conflict of Interest Policy, which prohibits related persons and others from having a financial interest in any competitor, customer, vendor or supplier of ours;
the related person’s role in arranging the transaction;
the potential for the transaction to be viewed as representing or leading to an actual or apparent conflict of interest; and
any other factors that our Corporate Governance Committee deems appropriate.
Our Code of Business Conduct, which sets forth legal and ethical guidelines for all of our directors and employees, states that directors, executive officers and employees must avoid relationships or activities that might impair their ability to make objective and fair decisions while acting in their Company roles. Other than the sponsored research agreement described below, thereThere are no relationships or transactions with related persons that are required to be disclosed in this Proxy Statement under SEC rules.
On January 27, 2017, we entered into a sponsored research agreement with Harvard University (the Research Agreement), under which the Artavanis-Tsakonas Laboratory at Harvard Medical School will conduct research to identify novel genes, targets and pathways that regulate neurodegenerative diseases. We believe the Research Agreement will support the identification of new drug targets and pathways in a resource efficient manner. Under the Research Agreement, we have an option to negotiate an exclusive license to any invention resulting from projects funded under the agreement. Dr. Spyros Artavanis-Tsakonas is the Principal Investigator and directs the activities of the Artavanis-Tsakonas Laboratory and is a Professor of Cell Biology at Harvard Medical School. Dr. Artavanis-Tsakonas served as a Visiting Scientist at Biogen from March 1, 2017 to February 28, 2018, which was a part-time position, and previously served as our Senior Vice President, Chief Scientific Officer. The Research Agreement requires us to make payments to Harvard University of $1.7 million per year, of which $1.0 million per year will directly fund the sponsored research at the Artavanis-Tsakonas Laboratory. The Research Agreement has an initial term of five years and may, after three years, be terminated on six months’ notice if certain milestones have not been met.
| | | | |
7164 | |  | |  |
| | |
76 | | Additional Information (continued) |
EQUITY COMPENSATION PLAN INFORMATION
The following table provides information as of December 31, 2017,2018, about:
the number of shares of common stock subject to issuance upon exercise of outstanding options and vesting of RSUs, MSUs and PSUs under plans adopted and assumed by us;
the weighted-average exercise price of outstanding options under plans adopted and assumed by us; and
the number of shares of common stock available for future issuance under our active plans: our 2017 Omnibus Equity Plan, ourNon-Employee Directors Equity Plan and our 2015 Employee Stock Purchase Plan.
| | Plan Category | | Number of Securities
to be Issued Upon
Exercise of
Outstanding Options and Rights
(a) | | Weighted-average
Exercise Price of
Outstanding
Options and Rights(1) (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column(a))(2) (c) | | Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights (a) | | Weighted-average Exercise Price of Outstanding Options and Rights(1) (b) | | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column(a))(2) (c) |
Equity compensation plans approved by stockholders | | 1,245,385 | | $ 53.83 | | 21,805,335 | | 1,308,264 | | $ 53.82 | | 20,564,534 |
Equity compensation plans not approved by stockholders | | — | | — | | — | | — | | — | | — |
Total | | 1,245,385 | | $ 53.83 | | 21,805,335 | | 1,308,264 | | $ 53.82 | | 20,564,534 |
| (1) | The weighted-average exercise price includes all outstanding stock options but does not include RSUs MSUs or PSUs, which do not have an exercise price. If the RSUs were included in this calculation, the weighted average exercise price would be $1.82. The total number of RSUs included in column (a) is 1,203,299. |
| (2) | Of these shares, (a) 15,185,03014,127,581 remain available for future issuance under our 2017 Omnibus Equity Plan, (b) 717,480703,425 remain available for future issuance under ourNon-Employee Directors Equity Plan and (c) 5,902,8255,733,528 remain available under our 2015 Employee Stock Purchase Plan. In addition to shares issuable upon the exercise of options or rights, the shares under our 2017 Omnibus Equity Plan and ourNon-Employee Directors Equity Plan may also be issued other than upon such exercise. |
| | | | |
7265 | |  | |  |
| | |
76 | | Additional Information (continued) |
MISCELLANEOUS
Stockholder Proposals
Stockholder proposals submitted pursuant to Exchange Act Rule14a-8 and intended to be presented at our 20192020 annual meeting of stockholders must be received by our Secretary no later than December 28, 2018,30, 2019, to be eligible for inclusion in our proxy statement and form of proxy relating to that meeting.
A stockholder proposal submitted outside the processes of Rule14a-8 and not for inclusion in our proxy statement for the 20192020 annual meeting of stockholders will be ineligible for presentation at the meeting unless the stockholder gives timely notice of the proposal in writing to our Secretary at our principal executive offices and otherwise complies with the provisions of our Bylaws. To be timely, our Bylaws provide that we must have received the stockholder’s notice no later than March 14, 2019,21, 2020, and no earlier than February 12, 2019.20, 2020. However, if the date of the 20192020 annual meeting of stockholders is more than 30 days before or more than 60 days after the first anniversary of the Annual Meeting, we must receive the stockholder’s notice not earlier than the close of business on the 120th day before the 20192020 annual meeting of stockholders and not later than the close of business on the later of (1) the 90th day before the 20192020 annual meeting of stockholders and (2) the 10th day following the day on which public announcement of the date of the 20192020 annual meeting of stockholders is first made.
All stockholder proposals for our 20192020 annual meeting of stockholders should be sent to our Secretary, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142.
Other Stockholder Communications
Generally, stockholders who have questions or concerns should contact our Investor Relations department at (781)(781) 464-2442. However, stockholders who wish to communicate directly with our Board of Directors, or any individual director, should direct questions in writing to our Secretary, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142. Communications addressed in this manner will be forwarded directly to our Board of Directors or named individual director(s).
Incorporation by Reference
Notwithstanding anything to the contrary set forth in any of our previous filings under the securities laws that might incorporate future filings, including this Proxy Statement, in whole or in part, the Compensation Committee Report, the Audit Committee Report, the content ofwww.biogen.com, including the charters of the committees of our Board of Directors, Corporate Governance Principles, Related Person Transaction Policy, Conflicts of Interest Policy, Code of Business Conduct and Bylaws, included or referenced in this Proxy Statement shall not be incorporated by reference into any such filings.
Copies of Annual Meeting Materials
Some banks, brokers and other nominee record holders may be participating in the practice of householding proxy statements and annual reports. This means that, unless you have instructed otherwise, only one copy of this Proxy Statement, Annual Report or Notice of Internet Availability of Proxy Materials, as applicable, may have been sent to multiple stockholders in your household.We will promptly deliver a separate copy of any of these documents without charge to you if you write or call Investor Relations, Biogen Inc., 225 Binney Street, Cambridge, Massachusetts 02142,(781) 464-2442.464-2442.If you want to receive separate copies of our proxy statement, annual report or Notice of Internet Availability of Proxy Materials, as applicable, in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker or other nominee record holder, or you may contact us at the above address or phone number.
Manner and Cost of Proxy Solicitation
Biogen pays the cost of soliciting proxies. In addition to solicitation by mail, our directors, officers and employees may contact you in person, by telephone or by email or other electronic means. None of our directors, officers or employees will receive additional compensation for soliciting you. We will reimburse brokerage houses, banks, custodians and other nominees and fiduciaries forout-of-pocket expenses incurred in forwarding our proxy solicitation materials to, and obtaining instructions relating to such materials from, beneficial owners of our common stock. Georgeson LLC, New York, New York, has been retained to assist us in the solicitation of proxies at a fee estimated not to exceed $11,000.
| | | | |
7366 | |  | |  |
APPENDIX A
GAAP toNon-GAAP Reconciliation
Diluted Earnings Per Share and Net Income Attributable to Biogen Inc.
(unaudited, $ in millions, except per share amounts)
| | | | | | | | | | | | For the Twelve Months Ended |
| | | For the Twelve Months Ended | |
| | | December 31, 2017 | | December 31, 2016 | | December 31, 2018 | | December 31, 2017 |
GAAP earnings per share – Diluted | | | $ | 11.92 | | | | $ | 16.93 | | | | $21.58 | | | | $11.92 | |
Adjustments to GAAP net income attributable to Biogen Inc. (as detailed below) | | | | 9.89 | | | | | 3.29 | | | | 4.62 | | | | 9.89 | |
Non-GAAP earnings per share – Diluted | | | $ | 21.81 | | | | $ | 20.22 | | | | $26.20 | | | | $21.81 | |
| | | | | | | | | | |
| | | For the Twelve Months Ended |
| | | December 31, 2017 | | December 31, 2016 |
GAAP net income attributable to Biogen Inc. | | | $ | 2,539.1 | | | | $ | 3,702.8 | |
Adjustments: | | | | | | | | | | |
Amortization of acquired intangible assetsA,B | | | | 814.7 | | | | | 373.6 | |
TECFIDERA litigation settlement chargeA | | | | — | | | | | 454.8 | |
Acquiredin-process research and development | | | | 120.0 | | | | | — | |
Loss (gain) on fair value remeasurement of contingent consideration | | | | 62.7 | | | | | 14.8 | |
Net distribution to noncontrolling interestsC | | | | 109.7 | | | | | — | |
Gain on deconsolidation of variable interest entities | | | | — | | | | | (4.4) | |
Hemophilia business separation costs | | | | 19.2 | | | | | 18.1 | |
Restructuring, business transformation and other cost saving initiatives: | | | | | | | | | | |
2017 corporate strategy implementationD | | | | 18.5 | | | | | — | |
Restructuring chargesD | | | | 0.9 | | | | | 33.1 | |
Cambridge manufacturing facility rationalization costsE | | | | — | | | | | 54.8 | |
Income tax effect related to reconciling items | | | | (213.0) | | | | | (224.9) | |
Tax reformF | | | | 1,173.6 | | | | | — | |
Non-GAAP net income attributable to Biogen Inc. | | | $ | 4,645.4 | | | | $ | 4,422.7 | |
| | | | | | | | | | |
| |
| | | For the Twelve Months Ended |
| | |
| | | December 31, 2018 | | December 31, 2017(1) |
GAAP net income attributable to Biogen Inc. | | | | $4,430.7 | | | | | $2,539.1 | |
Adjustments: | | | | | | | | | | |
Amortization of acquired intangible assetsA, B | | | | 747.3 | | | | | 814.7 | |
Acquiredin-process research and development | | | | 112.5 | | | | | 120.0 | |
Research and developmentC | | | | 10.0 | | | | | — | |
(Gain) loss on fair value remeasurement of contingent considerationD | | | | (12.3) | | | | | 62.7 | |
Premium paid on purchase of Ionis common stockE | | | | 162.1 | | | | | — | |
(Gain) loss on equity security investments | | | | (128.0) | | | | | — | |
Net distribution to noncontrolling interestsF | | | | 43.7 | | | | | 132.4 | |
Restructuring, business transformation and other cost saving initiatives: | | | | | | | | | | |
2017 corporate strategy implementationG | | | | 10.9 | | | | | 18.5 | |
Restructuring chargesG | | | | 12.0 | | | | | 0.9 | |
Hemophilia business separation costs | | | | — | | | | | 19.2 | |
Income tax effect related to reconciling items | | | | (146.6) | | | | | (235.7) | |
Elimination of deferred tax assetH | | | | 10.6 | | | | | — | |
Tax reformI | | | | 124.9 | | | | | 1,173.6 | |
Non-GAAP net income attributable to Biogen Inc. | | | | $5,377.8 | | | | | $4,645.4 | |
Free Cash Flow Reconciliation
(unaudited, $ in millions)
| | | | | |
| |
| | For the Twelve
Months Ended |
| | December 31, 2018 |
Net cash flows provided by operating activities | | | | $ 6,187.7 | |
Purchases of property, plant and equipment (Capital Expenditures) | | | | (770.6) | |
Contingent consideration related to Fumapharm AG acquisition | | | | (1,500.0) | |
Free Cash Flow | | | | $ 3,917.1 | |
| (1) | On February 1, 2017, we completed thespin-off of our hemophilia business. Our consolidated results of operations reflect the financial results of our hemophilia business through January 31, 2017. |
| A | Amortization of acquired intangible assets for the twelve months ended December 31,In January 2017 includes $444.2 million of impairmentwe entered into a settlement and amortization chargeslicense agreement among Biogen Swiss Manufacturing GmbH, Biogen International Holding Ltd., Forward Pharma A/S (Forward Pharma) and certain related parties, which was effective February 1, 2017. Pursuant to the intangible asset associated with ourthis
|
| | | | |
| A-1 | |  | |  |
Appendix A(continued)
| agreement, we obtained U.S. and rest of world licenses to Forward Pharma’s intellectual property, including Forward Pharma’s intellectual property related to TECFIDERA. In exchange, for these licenses, we paid Forward Pharma $1.25 billion in cash. During the fourth quartercash, of 2016 wewhich $795.2 million was recognized apre-tax charge of $454.8 million andas an intangible asset in the first quarter of 2017 we recognized intangible assets of $795.2 million related to this agreement.2017. |
| | We have two intellectual property disputes with Forward Pharma, one in the U.S. and one in the E.U.,European Union, concerning intellectual property related to TECFIDERA. |
| In March 2017 the U.S. intellectual property dispute was decided in our favor. Forward Pharma appealed to the U.S. Court of Appeals for the Federal Circuit. We evaluated the recoverability of the U.S. asset acquired from Forward Pharma and recorded ana $328.2 million impairment charge in the first quarter of 2017 to adjust the carrying value of the acquired U.S. asset to fair value reflecting the impact of the developments in the U.S. legal dispute. dispute and continued to amortize the remaining net book value of the U.S. intangible asset in our consolidated statements of income utilizing an economic consumption model. The U.S. Court of Appeals for the Federal Circuit upheld the U.S. Patent and Trademark Office’s March 2017 ruling and in January 2019 denied Forward Pharma’s petition for rehearing. We evaluated the recoverability of the U.S. asset based upon these most recent developments and recorded a $176.8 million impairment charge in the fourth quarter of 2018 to reduce the remaining net book value of the U.S. asset to zero. |
| In March 2018 the European Patent Office issued its decision revoking(EPO) revoked Forward Pharma’s European Patent No. 2 801 355. Forward Pharma has filed an appeal to the Technical Boards of Appeal of the EPO and the appeal is pending. Based upon our assessment of these rulings,this ruling, we continue to amortize the remaining net book value of the U.S. and rest of world intangible assetsasset in our consolidated statements of income utilizing an economic consumption model. |
| | The TECFIDERA litigation settlement charge for the twelve months ended December 31, 2016, represents the portion of the $1.25 billion cash payment made in the first quarter of 2017 attributable to our sales of TECFIDERA during the period April 2014 through December 31, 2016. |
B | Amortization of acquired intangible assets for the twelve months ended December 31, 2017, also includes a $31.2 millionpre-tax impairment charge related to our acquired andin-licensed rights and patents intangible asset dueassociated with ZINBRYTA after the initiation of an European Medicines Agency review (referred to as an Article 20 Procedure) of ZINBRYTA following the report of a case of fatal fulminant liver failure, as well as four cases of serious liver injury. |
| B | Amortization of acquired intangible assets for the twelve months ended December 31, 2018, includes the impact of impairment charges totaling $189.3 million related to certainin-process research and development (IPR&D) assets associated with our vixotrigine (BIIB074) program. |
| During the third quarter of 2018 we completed a Phase 2b study of vixotrigine for the treatment of painful lumbosacral radiculopathy (PLSR). The study did not meet its primary or secondary efficacy endpoints; therefore, we discontinued development of vixotrigine for the treatment of PLSR and we recognized an impairment charge of approximately $60.0 million during the third quarter of 2018 to reduce the fair value of the related IPR&D intangible asset to zero. In addition, we delayed the initiation of the Phase 3 studies of vixotrigine for the treatment of trigeminal neuralgia (TGN) as we awaited the outcome of ongoing interactions with the U.S. Food and Drug Administration (FDA) regarding the design of the Phase 3 studies, a more detailed review of the data from the Phase 2b study of vixotrigine for the treatment of PLSR and insights from the Phase 2 study of vixotrigine for the treatment of small fiber neuropathy. We reassessed the fair value of our vixotrigine program for the treatment of TGN using reduced expected lifetime revenues, higher expected clinical development costs and a lower cumulative probability of success and, as a result of that assessment, we recognized an impairment charge of $129.3 million during the third quarter of 2018 to reduce the fair value of the IPR&D intangible asset associated with our vixotrigine program for the treatment of TGN to $41.8 million. |
| C | GAAP research and development expense for the twelve months ended December 31, 2018, include a $10.0 million contingent consideration payment accrued in relation to the Article 20 Procedureacquisition of ZINBRYTA.an asset. |
| D | During the third quarter of 2018, we adjusted the fair value of our contingent consideration obligations related to our vixotrigine program for the treatment of TGN to reflect the lower cumulative probabilities of success, which resulted in a gain of $89.6 million. |
| In late December 2018 we received feedback from the FDA regarding the design of the Phase 3 studies of vixotrigine for the treatment of TGN. Following this feedback, we are now planning to initiate the Phase 3 studies for our vixotrigine program for the treatment of TGN and, as a result, we adjusted the fair value of our contingent consideration obligations related to our vixotrigine program for the treatment of TGN to reflect increased probabilities of success and recognized a loss of $80.6 million in the fourth quarter of 2018. |
| E | In June 2018 we closed a newten-year exclusive agreement with Ionis Pharmaceuticals, Inc. (Ionis) to develop novel antisense oligonucleotide drug candidates for a broad range of neurological diseases for a total payment of $1.0 billion consisting of an upfront payment of $375.0 million and the purchase of approximately 11.5 million shares of Ionis’ common stock at a cost of $625.0 million. |
| The 11.5 million shares of Ionis’ common stock were purchased at a premium to their fair value at the transaction closing date. The premium consisted of acquiring the shares at a price above the fair value based on the trailing10-day weighted-average close price prior to entering into the agreement in April 2018 and the effect of certain holding period restrictions. We recorded an asset of $462.9 million in investments and other assets in our consolidated balance sheets reflecting the fair value of the common stock as of the purchase date and a charge of $162.1 million to research and development expense in our consolidated statements of income during the second quarter of 2018 reflecting the premium paid for the common stock. |
| | | | |
A-1A-2 | |  | |  |
Appendix A(continued)
CF | In October 2017 we amended the terms of our collaboration and license agreement with Neurimmune SubOne AG (Neurimmune). Under the amended agreement, we made a $150.0 million payment to Neurimmune in exchange for a 15% reduction in the previously negotiated royalty rates payable on products developed under this agreement. In May 2018 we made an additional $50.0 million payment to Neurimmune to further reduce the previously negotiated royalty rates payable on products developed under this agreement by an additional 5%. |
| Net distribution to noncontrolling interestsinterest for the twelve months ended December 31, 2018, reflects the $50.0 million payment made to Neurimmune, net of Neurimmune’s tax, in May 2018. |
| Net distribution to noncontrolling interest for the twelve months ended December 31, 2017, reflects theafter-tax $150.0 million upfront payment made to Neurimmune, net of Neurimmune’s tax, in exchange for a 15% reduction in royalty rates payable on potential commercial sales of aducanumab. This upfront payment is in relation to the amendment of the terms of our collaboration agreement with Neurimmune.October 2017. |
DG | 2017 corporate strategy implementation and restructuring charges for the twelve months ended December 31, 2017, are related to our efforts to create a leaner and simpler operating model. |
| H | Restructuring charges for the twelve months ended December 31, 2016, include $8.0 millionElimination of costs incurred in connection with our 2015 corporate restructuring. Restructuring charges for the twelve months ended December 31, 2016, include charges of $17.7 million incurred in connection with our 2016 restructuring resulting from our decisiondeferred tax asset due tospin-off our hemophilia business. Restructuring charges for the twelve months ended December 31, 2016, also include severance charges of $7.4 million related to employee separation costs Samsung Bioepis Co., Ltd. qualifying as a result of our decision to vacate and cease manufacturing in Cambridge, MA and vacate our warehouse in Somerville, MA. |
E | Cambridge manufacturing facility rationalization costscorporate joint venture for the twelve months ended December 31, 2016, reflect $45.5 million of additional depreciation expense included in cost of sales, excluding amortization of acquired intangible assets in our condensed consolidated statements of income. Cambridge manufacturing facility rationalization costs for the twelve months ended December 31, 2016, also include charges of $6.9 million for the write-down of excess inventory.accounting purposes.
|
FI | On December 22,The Tax Cuts and Jobs Act of 2017 the 2017(2017 Tax Act was signed into law and hasAct) resulted in significant changes to the U.S. corporate income tax system. The 2017 Tax Act includesThese changes include a federal statutory rate reduction from 35% to 21%, the elimination or reduction of certain domestic deductions and credits the transition of U.S. international taxation from a worldwide tax system towards a territorial tax system,and limitations on the deductibility of interest expense and executive compensationcompensation. The 2017 Tax Act also transitions international taxation from a worldwide system to a modified territorial system and base-erosionincludes base erosion prevention measures on futurenon-U.S. earnings, of U.S. entities, which has the effect of subjecting certain earnings of our earnings of foreign subsidiaries to U.S. taxation. These changes are effective beginning in 2018.taxation as globallow-taxed income (GILTI). During the fourth quarter of 2018 we elected to recognize deferred taxes for basis differences expected to reverse as GILTI is incurred and have established initial deferred tax balances, as of the enactment date of the 2017 Tax Act.
|
| | TheDuring the fourth quarter of 2017 Tax Act also includeswe recognized within our provision for income taxes a $1.2 billion provisional estimate pursuant to U.S. Securities and Exchange Commission Staff Accounting Bulletin No. 118. Our provisional estimate included an amount of $989.6 million associated with aone-time mandatory deemed repatriation tax on accumulated foreign subsidiaries’ previously untaxed foreign earnings (the Transition Toll Tax). and $184.0 million related to the impact of remeasuring our deferred tax balances to reflect the new federal statutory rate and other changes to U.S. tax law. |
| | Changes in tax rates and tax laws are accountedTax reform amounts for in the period of enactment. Therefore, during the yeartwelve months ended December 31, 2017, we recorded a charge totaling $1,173.62018, reflect the effect of an expense of $135.8 million related to the establishment of GILTI deferred taxes. |
| Tax reform amounts for the twelve months ended December 31, 2018, also reflect the effect of a net reduction of $34.6 million to our current2017 preliminary Transition Toll Tax estimate, an expense of $12.7 million for the provisionsremeasurement of our deferred tax balance and an $11.0 million expense to reflect other aspects of the 2017 Tax Act, including a $989.6 million expense underAct. |
| The final determination of the Transition Toll Tax. The Transition Toll Tax must be paid over an eight-year period, startingand remeasurement of our deferred assets and liabilities was completed in 2018, and will not accrue interest.the fourth quarter of 2018. |
Use ofNon-GAAP Financial Measures
We supplement our consolidated financial statements presented on a GAAP basis by providing additional measures which may be considered“Non-GAAP” financial measures under applicable SEC rules. We believe that the disclosure of theseNon-GAAP financial measures provides additional insight into the ongoing economics of our business and reflects how we manage our business internally, set operational goals and formsform the basis of our management incentive programs. TheseNon-GAAP financial measures are not in accordance with generally accepted accounting principles in the United States and should not be viewed in isolation or as a substitute for reported, or GAAP, net income attributable to Biogen Inc. and, diluted earnings per share.share and net cash flows provided by operating activities.
Our“Non-GAAP net income attributable to Biogen Inc.” and“Non-GAAP earnings per share – Diluted” financial measures exclude the following items from “GAAP net income attributable to Biogen Inc.” and “GAAP earnings per share – Diluted”:
1. Purchase accounting, merger-related and merger-relatedother adjustments
We exclude certain purchase accounting related items associated with the acquisition of businesses, assets and amounts in relation to the consolidation or deconsolidation of variable interest entities for which we are the primary beneficiary. These adjustments include, but are not limited to, charges forin-process research IPR&D and development,certain milestones, the amortization of certain acquired intangible assets and charges or credits from the fair value remeasurement of our contingent consideration obligations.
| | | | |
| A-3 | |  | |  |
Appendix A(continued)
2. Hemophilia business separation costs
We have excluded costs that are directly associated with the set up andspin-off of our hemophilia business into an independent, publicly-traded company on February 1, 2017. These costs represent incremental third partythird-party costs attributable solely to hemophilia separationspin-off and set up activities.
| | | | |
A-2 | |  | |  |
Appendix A(continued)
3. Restructuring, business transformation and other cost saving initiatives
We exclude costs associated with the Company’sour execution of certain strategies and initiatives to streamline operations, achieve targeted cost reductions, rationalize manufacturing facilities or refocus R&D activities. These costs may include employee separation costs, retention bonuses, facility closing and exit costs, asset impairment charges or additional depreciation when the expected useful life of certain assets have been shortened due to changes in anticipated usage and other costs or credits that management believes do not have a direct correlation to ouron-going ongoing or future business operations.
4. (Gain) loss on equity security investments
Effective January 2018 we exclude unrealized and realized gains and losses and discounts or premiums on our equity security investments as we do not believe that these components of income or expense have a direct correlation to our ongoing or future business operations.
5. Other items
We evaluate other items of income and expense on an individual basis and consider both the quantitative and qualitative aspects of the item, including (i) its size and nature, (ii) whether or not it relates to our ongoing business operations and (iii) whether or not we expect it to occur as part of our normal business on a regular basis. We also include an adjustment to reflect the related tax effect of all reconciling items within our reconciliation of our GAAP toNon-GAAP net income attributable to Biogen Inc. and diluted earnings per share.
“Free Cash Flow” is defined as net cash flows provided by operating activities less purchases of property, plant and equipment and contingent consideration related to our acquisition of Fumapharm AG as disclosed in our 2018 Annual Report on Form10-K.
| | | | |
A-3A-4 | |  | |  |


BIOGEN INC.
225 BINNEY STREET
CAMBRIDGE, MA 02142
VOTE BY INTERNET
Before The Meeting- Go towww.proxyvote.com
Use the Internet to transmit your voting instructions and for electronic delivery of information up until 11:59 P.M. Eastern Time the day before the meeting date. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form.
During The Meeting - Go towww.virtualshareholdermeeting.com/BIIB2018BIIB2019
You may attend the Meeting via the Internet and vote during the Meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions.
VOTE BY PHONE -1-800-690-6903
Use any touch-tone telephone to transmit your voting instructions up until 11:59 P.M. Eastern Time the day before the meeting date. Have your proxy card in hand when you call and then follow the instructions.
VOTE BY MAIL
Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717.
| | |
TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: | | E43520-P06571E76108-P22124 KEEP THIS PORTION FOR YOUR RECORDS |
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — |
DETACH AND RETURN THIS PORTION ONLY
THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| BIOGEN INC. | | | | | | | | | | |
| | | | | | | | | | |
| | The Board recommends a voteFOR the following proposals: | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 1. | | Election of Directors. To elect the elevenfourteen director nominees numbered 1a through 1k1n to serve for aone-year term extending until the 20192020 annual meeting of stockholders and their successors are duly elected and qualified. | | For | | Against | | Abstain | | | | | | | | | | | | For | | Against | | Abstain | | |
| | | | | | | | | | | | | | | |
| | | | 1a. 1b. 1c. 1d. 1e. 1f. 1g. 1h. 1i. | | John R. Chiminski Alexander J. Denner Caroline D. Dorsa William A. Hawkins Nancy L. Leaming Jesus B. Mantas Richard C. Mulligan Robert W. Pangia Stelios Papadopoulos Brian S. Posner
Eric K. Rowinsky
Lynn Schenk
| | ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ ☐ | | | | 1j. 1k. 1l. 1m. 1n. | | Brian S. Posner Eric K. Rowinsky Lynn Schenk Stephen A. Sherwin Michel Vounatsos | | | | ☐ | | ☐ | | ☐ | | | | |
| | | | | | | | | | | 1k.☐
| | Michel Vounatsos☐
| | | | ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ | | ☐ ☐ ☐ ☐ ☐ | | | | |
| | | | | | | | | 2. | | To ratify the selection of PricewaterhouseCoopers LLP as Biogen Inc.’s independent registered public accounting firm for the fiscal year ending December 31, 2018.2019. | | ☐ | | ☐ | | ☐ | | | | |
| | | | | | |
| | | | 3. | | Say on Pay - To approve an advisory vote on executive compensation. | | ☐ | | ☐ | | ☐ | | | | |
| | | | | | | | | The Board recommends a voteAGAINST the following stockholder proposals:
| | | | | | | | | | | | |
| | | | | | | | | 4.
| | Stockholder proposal requesting certain proxy access bylaw amendments.
| | | | ☐
| | ☐
| | ☐
| | | | |
| | | | | | | | | | | | | | 5.
| | Stockholder proposal requesting a report on the extent to which risks related to public concern over drug pricing strategies are integrated into incentive compensation arrangements.
| | ☐
| | ☐
|
| | ☐
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| | Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | Signature [PLEASE SIGN WITHIN BOX] | | | | Date | | | | | | | | | | Signature (Joint Owners) | | Date | | | | | | |
Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting:
The 20182019 Notice and Proxy Statement and 20172018 Annual Report with Form10-K are available
at:www.proxyvote.com.
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
E43521-P06571E76109-P22124
BIOGEN INC.
Annual Meeting of Stockholders
June 12, 2018,19, 2019, 9:00 a.m. Eastern Time
This proxy is solicited by the Board of Directors
The undersigned hereby appoints Michel Vounatsos, Jeffrey D. Capello and Susan H. Alexander, and each of them (with full power to act alone), as proxies of the undersigned with all the powers the undersigned would possess if present during the 20182019 Annual Meeting,and with full power of substitution in each of them to appear, represent and vote all shares of common stock of Biogen Inc. which the undersigned would be entitled to vote at the 20182019 Annual Meeting of Stockholders, to be held at Biogen Inc.’s offices located at 225 Binney Street, Cambridge, Massachusetts 02142 and online at www.virtualshareholdermeeting.com/BIIB2018BIIB2019 on Tuesday,Wednesday, June 12, 2018,19, 2019, at 9:00 a.m. Eastern Time, and at any adjournment or postponement thereof.
The shares represented by this proxy will be voted as directed herein. If no direction is indicated, such shares will be voted FOR the election of all of the director nominees listed in Proposal 1 and FOR Proposals 2 and 3 and AGAINST Proposals 4 and 5.3. As to any other matter that may be properly brought before the meeting or any adjournment or postponement thereof, proxy holders will vote in accordance with their best judgment.
Continued and to be signed on reverse side




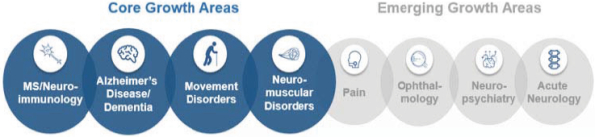









 By Internet. You may vote atwww.proxyvote.com, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted throughwww.proxyvote.com must be received by 11:59 p.m. Eastern Time on June
By Internet. You may vote atwww.proxyvote.com, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted throughwww.proxyvote.com must be received by 11:59 p.m. Eastern Time on June 
 By Telephone. You may vote using a touch-tone telephone by calling1-800-690-6903, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted by telephone must be received by 11:59 p.m. Eastern Time on June
By Telephone. You may vote using a touch-tone telephone by calling1-800-690-6903, 24 hours a day, seven days a week. You will need the16-digit control number included on your Notice of Internet Availability of Proxy Materials or, if you received a printed copy of the proxy materials, on your proxy card. Votes submitted by telephone must be received by 11:59 p.m. Eastern Time on June 
 By Mail.If you received printed proxy materials, you may submit your vote by completing, signing and dating each proxy card received and returning it in the prepaid envelope. Sign your name exactly as it appears on the proxy card. Proxy cards submitted by mail must be received no later than June
By Mail.If you received printed proxy materials, you may submit your vote by completing, signing and dating each proxy card received and returning it in the prepaid envelope. Sign your name exactly as it appears on the proxy card. Proxy cards submitted by mail must be received no later than June 
 During the Annual Meeting. You may vote during the Annual Meeting by submitting a written ballot in person at the Annual Meeting. To obtain directions to attend the Annual Meeting, please contact our Investor Relations department at (781)464-2442. We will pass out ballots at the Annual Meeting to anyone who wishes to vote in person.
During the Annual Meeting. You may vote during the Annual Meeting by submitting a written ballot in person at the Annual Meeting. To obtain directions to attend the Annual Meeting, please contact our Investor Relations department at (781)464-2442. We will pass out ballots at the Annual Meeting to anyone who wishes to vote in person.

































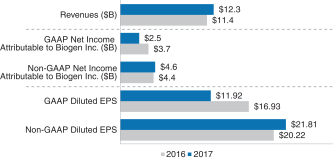

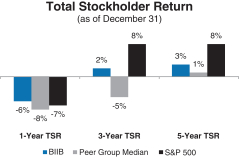






 Target Setting
Target Setting


 Monitoring & Tracking
Monitoring & Tracking
 Results & Awards:
Results & Awards:








